The more commonly used measure of antidepressant efficacy in clinical trials has been a 50% reduction from baseline total scores on the Hamilton Rating Scale for Depression (HRSD) (Reference Prien, Carpenter and KupferPrien et al, 1991; Depression Guideline Panel, 1993). A more stringent measure of antidepressant efficacy is the ability to induce remission, a clinical state characterised by minimal residual symptoms (e.g. 17-item HRSD total scores of ≤ 7; Reference Frank, Prien and JarrettFrank et al, 1991). Patients treated to full remission are less likely to relapse (Reference Thase, Simons and McGearyThase et al, 1992; Reference Fava, Grandi and ZieleznyFava et al, 1996) and have more normal psychosocial and vocational functioning (Reference Miller, Keitner and SchatzbergMiller et al, 1998) when compared with incompletely remitted patients. This report presents the results of a pooled analysis of remission rates comparing venlafaxine and three selective serotonin reuptake inhibitors (SSRIs): fluoxetine, paroxetine and fluvoxamine. It includes original data from 2045 patients with depression, drawn from eight related randomised controlled trials. We undertook this analysis to test the hypothesis that patients treated with venlafaxine, a serotonin-noradrenaline reuptake inhibitor (SNRI) (Reference Muth, Haskins and MoyerMuth et al, 1986), are significantly more likely to achieve remission than those treated with SSRIs.
METHOD
This analysis included data from the patients with depression who participated in the eight double-blind, randomised clinical trials comparing venlafaxine and SSRIs conducted by the Clinical Research and Development department at Wyeth-Ayerst Laboratories during the development of the immediate-release (IR) and extended-release (XR) formulations of venlafaxine. Results from four of these studies have been published (Reference Clerc, Ruimy and Verdeau-PaillèsClerc et al, 1994; Reference Dierick, Ravizza and RealiniDierick et al, 1996; Silverstone et al, 1998; Reference Rudolph and FeigerRudolph & Feiger, 1999). Results from two studies have been presented as posters and published in abstract form (Reference SalinasSalinas et al, 1997; Reference Rudolph, Entsuah and AguiarRudolph et al, 1998a ). The remaining two studies are unpublished (Studies 347 and 349; data on file, Wyeth-Ayerst Laboratories, Philadelphia, PA). The doses employed were: venlafaxine IR, 75-375 mg/day; venlafaxine XR, 75-225 mg/day; fluoxetine, 20-80 mg/day; paroxetine, 20-40 mg/day; and fluvoxamine, 100-200 mg/day. Four studies included a placebo control group (Reference SalinasSalinas et al, 1997; Reference Rudolph, Entsuah and AguiarRudolph et al, 1998a ; Silverstone et al, 1998; Reference Rudolph and FeigerRudolph & Feiger, 1999). Each study was approved by the ethics committees of the participating sites and conducted according to the guidelines of the Declaration of Helsinki and its amendments. All patients provided written informed consent. Table 1 summarises the study characteristics.
Table 1 Studies pooled for analysis of the Hamilton Rating Scale for Depression remission (n=8)
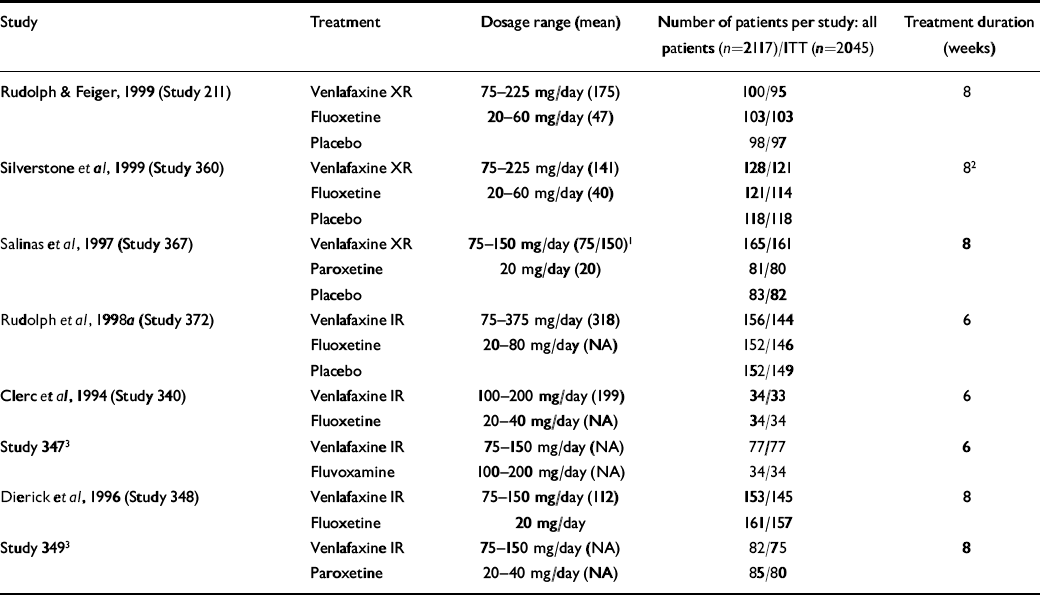
| Study | Treatment | Dosage range (mean) | Number of patients per study: all patients (n=2117)/ITT (n=2045) | Treatment duration (weeks) |
|---|---|---|---|---|
| Reference Rudolph and FeigerRudolph & Feiger, 1999 (Study 211) | Venlafaxine XR | 75-225 mg/day (175) | 100/95 | 8 |
| Fluoxetine | 20-60 mg/day (47) | 103/103 | ||
| Placebo | 98/97 | |||
| Silverstone et al, 1999 (Study 360) | Venlafaxine XR | 75-225 mg/day (141) | 128/121 | 82 |
| Fluoxetine | 20-60 mg/day (40) | 121/114 | ||
| Placebo | 118/118 | |||
| Reference SalinasSalinas et al, 1997 (Study 367) | Venlafaxine XR | 75-150 mg/day (75/150)1 | 165/161 | 8 |
| Paroxetine | 20 mg/day (20) | 81/80 | ||
| Placebo | 83/82 | |||
| Reference Rudolph, Entsuah and AguiarRudolph et al, 1998a (Study 372) | Venlafaxine IR | 75-375 mg/day (318) | 156/144 | 6 |
| Fluoxetine | 20-80 mg/day (NA) | 152/146 | ||
| Placebo | 152/149 | |||
| Reference Clerc, Ruimy and Verdeau-PaillèsClerc et al, 1994 (Study 340) | Venlafaxine IR | 100-200 mg/day (199) | 34/33 | 6 |
| Fluoxetine | 20-40 mg/day (NA) | 34/34 | ||
| Study 3473 | Venlafaxine IR | 75-150 mg/day (NA) | 77/77 | 6 |
| Fluvoxamine | 100-200 mg/day (NA) | 34/34 | ||
| Reference Dierick, Ravizza and RealiniDierick et al, 1996 (Study 348) | Venlafaxine IR | 75-150 mg/day (112) | 153/145 | 8 |
| Fluoxetine | 20 mg/day | 161/157 | ||
| Study 3493 | Venlafaxine IR | 75-150 mg/day (NA) | 82/75 | 8 |
| Paroxetine | 20-40 mg/day (NA) | 85/80 |
Patients
Patients could be enrolled if they were at least 18 years old and met the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM; American Psychiatric Association, 1987, 1994) for major depression (DSM-III-R) or major depressive disorder (DSM-IV) for at least 1 month. There were 68 in-patients (one study, Reference Clerc, Ruimy and Verdeau-PaillèsClerc et al, 1994) and 1977 out-patients; all patients had minimum scores of either 20 on the HRSD21 (Reference HamiltonHamilton, 1960) or 25 on the Montgomery-Åsberg Depression Rating Scale (MADRS; Reference Montgomery and ÅsbergMontgomery & Åsberg, 1979) at both pre-study and baseline (study day ‒1), with no greater than a 20% decrease in severity between pre-study and baseline evaluations.
Patients with clinically significant cardiovascular, renal or hepatic disease, seizure disorders, a recent history of alcohol or drug misuse or clinically significant abnormalities on baseline physical examination, electrocardiogram (ECG) or laboratory tests were excluded from participation. Patients who were hypersensitive to the study drugs or had used any investigational or antipsychotic drug within 30 days, a monoamine oxidase inhibitor within 14 days or other antidepressant, anxiolytic, sedative-hypnotic or non-psychopharmacological drugs with psychotropic effects within 7 days of double-blind treatment also were excluded. Chloral hydrate (maximum 2000 mg) or temazepam (20 mg; one study) were permitted as hypnotics. Table 2 summarises the socio-demographic and pre-treatment clinical characteristics of the pooled study groups.
Table 2 Baseline characteristics of intent-to-treat patients (pooled studies, n=2045)
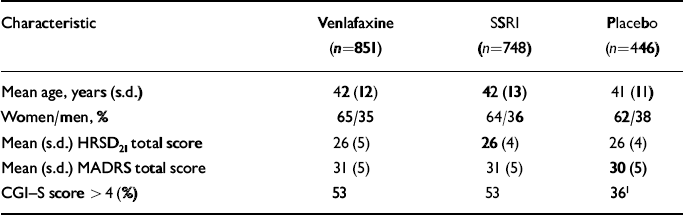
| Characteristic | Venlafaxine (n=851) | SSRI (n=748) | Placebo (n=446) |
|---|---|---|---|
| Mean age, years (s.d.) | 42 (12) | 42 (13) | 41 (11) |
| Women/men, % | 65/35 | 64/36 | 62/38 |
| Mean (s.d.) HRSD21 total score | 26 (5) | 26 (4) | 26 (4) |
| Mean (s.d.) MADRS total score | 31 (5) | 31 (5) | 30 (5) |
| CGI—S score > 4 (%) | 53 | 53 | 361 |
Study drugs
Patients were randomly assigned to treatment with venlafaxine (n=865), an SSRI (fluoxetine, n=563; paroxetine, n=160; or fluvoxamine, n=34) or placebo (four studies only, n=450) during the double-blind treatment period at the daily dosages shown in Table 1.
Efficacy and safety assessments
The HRSD, MADRS and Clinical Global Impression — Severity of Illness (CGI-S) (National Institute of Mental Health, 1985) were performed at study day ‒1, prior to double-blind therapy. These measures (along with the CGI improvement score) were reassessed on study days 7, 14, 21, 28, 42 and, if available, 56. Remission was defined as a total score of ≤7 on the first 17 items of the HRSD (Reference Frank, Prien and JarrettFrank et al, 1991).
Safety and tolerability were evaluated on the basis of adverse events that were recorded throughout the study evaluation period and changes that occurred in the physical examination, vital signs, 12-lead ECG recordings and clinical laboratory tests during treatment. For this report, only the proportions of patients withdrawn from double-blind therapy because of side-effects and lack of efficacy were compared.
Statistical analyses
The analyses were performed on data from a modified intent-to-treat sample, which included all patients who received at least one dose of study medication and had at least one HRSD evaluation during therapy. Remission rates were calculated using the last-observation-carried-forward (LOCF) method, which allowed the inclusion of patients who were withdrawn early. Pairwise comparisons of remission rates were made with Fisher's exact test. All tests of hypotheses were two-sided. Results of statistical analyses were considered significant when P was ≤0.05. The 95% confidence intervals (CIs) for differences in remission rates between groups were calculated for the pooled data at each interval. The odds ratios for remission with a 95% CI (Reference RothmanRothman, 1986) were also calculated for venlafaxine or an SSRI v. placebo and for venlafaxine v. the SSRIs. Homogeneity of the odds ratios across studies was tested with the Breslow—Day test (Reference Breslow and DayBreslow & Day, 1980).
Analyses of various subgroups were performed to corroborate the overall findings, including studies using the extended-release or immediate-release formulations, active-controlled studies, placebo-controlled studies, the single inpatient study, the seven out-patient studies and studies utilising fluoxetine v. those using other SSRIs. Additional analyses compared alternative definitions of remission to ensure the robustness of the findings. The following additional definitions were examined: HRSD21 ≤7, HRSD21 ≤8, HRSD21 ≤10, HRSD17 ≤10 plus CGI=1, MADRS <10, and ≥50% decrease from baseline HRSD21 scores. Finally, a sensitivity analysis was performed by removing each individual study from the pooled analysis, one at a time (Reference Thase, Greenhouse and FrankThase et al, 1997).
RESULTS
Among the 2117 patients enrolled, 2045 (96.6%) were included in the intent-to-treat analyses of venlafaxine IR and venlafaxine XR (n=851), the SSRIs (n=748) and placebo (n=446). Results from one investigational site (27 patients in total) were excluded prior to the analysis because the validity of the data could not be verified. The treatment groups had similar characteristics at baseline (see Table 2). However, patients enrolled in the four placebo-controlled studies were significantly less severely depressed than those enrolled in the other studies.
Final remission rates were 45% for venlafaxine (382/851), 35% for the SSRIs (260/748) and 25% for placebo (110/446). The differences for venlafaxine v. SSRIs, venlafaxine v. placebo and SSRIs v. placebo were highly statistically significant (P <0.001 for all comparisons).
Week-by-week comparisons are illustrated in Fig. 1. Venlafaxine was statistically significantly more effective than the SSRIs from week 2 onwards and versus placebo from week 3 onwards. The SSRI group had a significantly higher remission rate than the placebo group from week 4 onwards.
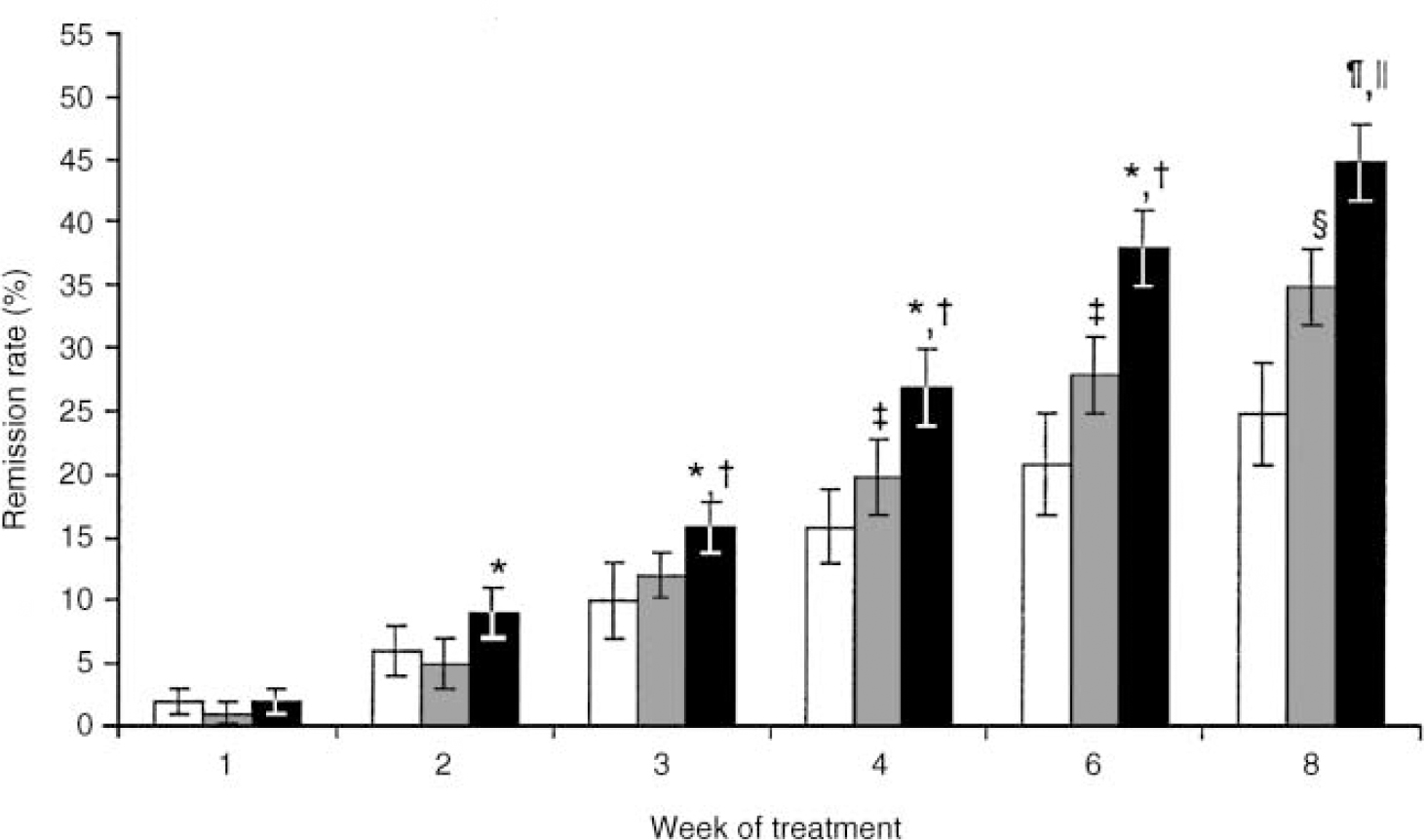
Fig. 1 Remission rates (HRSD17 score ≤7 ± 95% Cl) for pooled studies comparing venlafaxine (black bar), SSRI (grey bar) and placebo (white bar) treatments: * P ≤0.05, venlafaxine v. SSRI; † P ≤0.05, venlafaxine v. placebo; ‡ P ≤0.05, SSRI v. placebo; § P <0.001, SSRI v. placebo; ¶ P <0.001, venlafaxine v. SSRI; ∥ P <0.001, venlafaxine v. placebo. HRSD17, 17-item Hamilton Rating Scale for Depression; SSRI, selective serotonin reuptake inhibitor.
The results of the eight individual studies are summarised in Table 3. Odds ratios for remission ranged from 1.0 to 3.5, with an overall odds ratio of 1.5 (95% CI 1.3-1.9). Thus, there was a 50% greater chance of remission during venlafaxine treatment than during SSRI treatment. Testing for homogeneity of the odds ratios revealed no significant difference (χ2=8.63, d.f.=7, P=0.28). The sensitivity analysis similarly found that the significant difference between venlafaxine and the SSRIs was not dependent on any one study.
Table 3 Remission rates (%) and odds ratios for comparison of intent-to-treat 17-item Hamilton Rating Scale for Depression (HRSD17) remission by treatment1
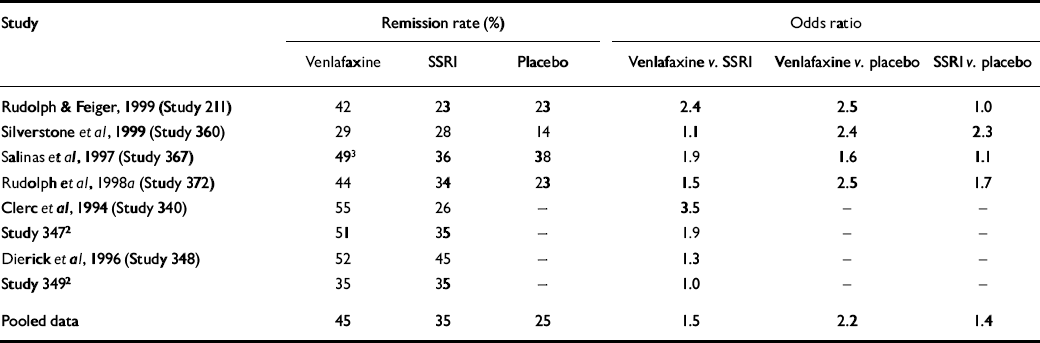
| Study | Remission rate (%) | Odds ratio | ||||
|---|---|---|---|---|---|---|
| Venlafaxine | SSRI | Placebo | Venlafaxine v. SSRI | Venlafaxine v. placebo | SSRI v. placebo | |
| Reference Rudolph and FeigerRudolph & Feiger, 1999 (Study 211) | 42 | 23 | 23 | 2.4 | 2.5 | 1.0 |
| Silverstone et al, 1999 (Study 360) | 29 | 28 | 14 | 1.1 | 2.4 | 2.3 |
| Reference SalinasSalinas et al, 1997 (Study 367) | 493 | 36 | 38 | 1.9 | 1.6 | 1.1 |
| Reference Rudolph, Entsuah and AguiarRudolph et al, 1998a (Study 372) | 44 | 34 | 23 | 1.5 | 2.5 | 1.7 |
| Reference Clerc, Ruimy and Verdeau-PaillèsClerc et al, 1994 (Study 340) | 55 | 26 | - | 3.5 | - | - |
| Study 3472 | 51 | 35 | - | 1.9 | - | - |
| Reference Dierick, Ravizza and RealiniDierick et al, 1996 (Study 348) | 52 | 45 | - | 1.3 | - | - |
| Study 3492 | 35 | 35 | - | 1.0 | - | - |
| Pooled data | 45 | 35 | 25 | 1.5 | 2.2 | 1.4 |
Figure 2 illustrates the results for various subgroup comparisons. The differences between venlafaxine and the SSRIs were statistically significant for all but one of the subgroup analyses. The comparison of venlafaxine and SSRI that included only the four studies that were not placebocontrolled was not statistically significant (P=0.055).
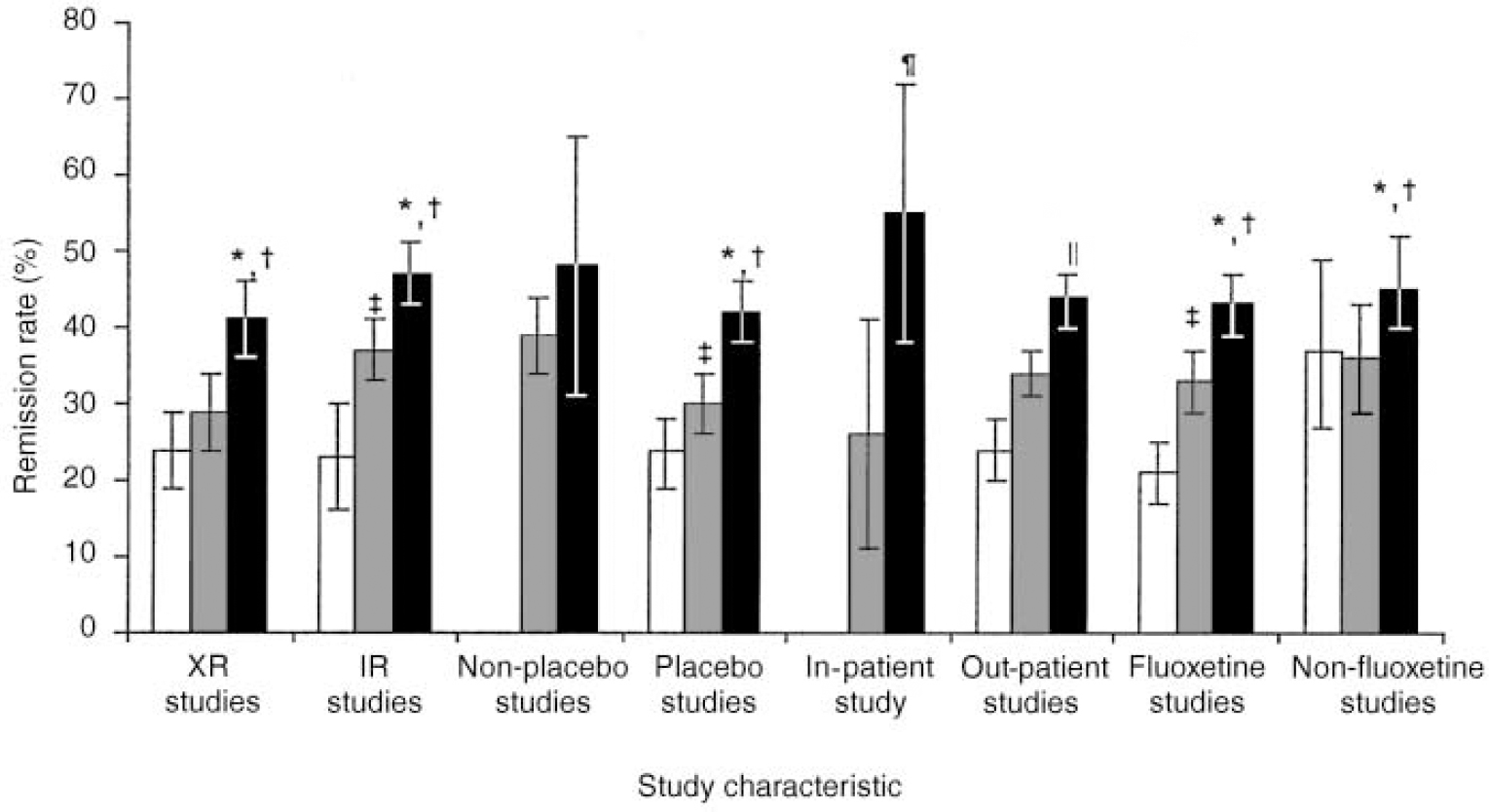
Fig. 2 Remission rates (HRSD17 score ≤ 7; ± 95% Cl) in different study types: *P=0.0009 (XR studies), P=0.003 (immediate-release studies) and P=0.0003 (placebo studies) (white bar), venlafaxine (black bar) v. SSRI (grey bar); †P < 0.001 (XR studies) and P < 0.0001 (immediate-release studies, placebo studies), venlafaxine v. placebo (white bar); ‡ P=0.028 (immediate-release studies, placebo studies), SSRI v. placebo; §P=0.055, venlafaxine v. SSRI; ¶ P=0.026 (in-patient study), venlafaxine v. SSRI; ∥ P=0.002 (out-patient studies), venlafaxine v. SSRI. HRSD17, 17-item Hamilton Rating Scale for Depression; XR, venlafaxine extended-release formulation; IR, venlafaxine immediate-release (conventional) formulation; SSRI, selective serotonin reuptake inhibitor.
Figure 3 summarises the results according to multiple alternative outcome criteria. Regardless of the definition used, venlafaxine was significantly more effective than the SSRIs, and the SSRIs were significantly more effective than placebo.
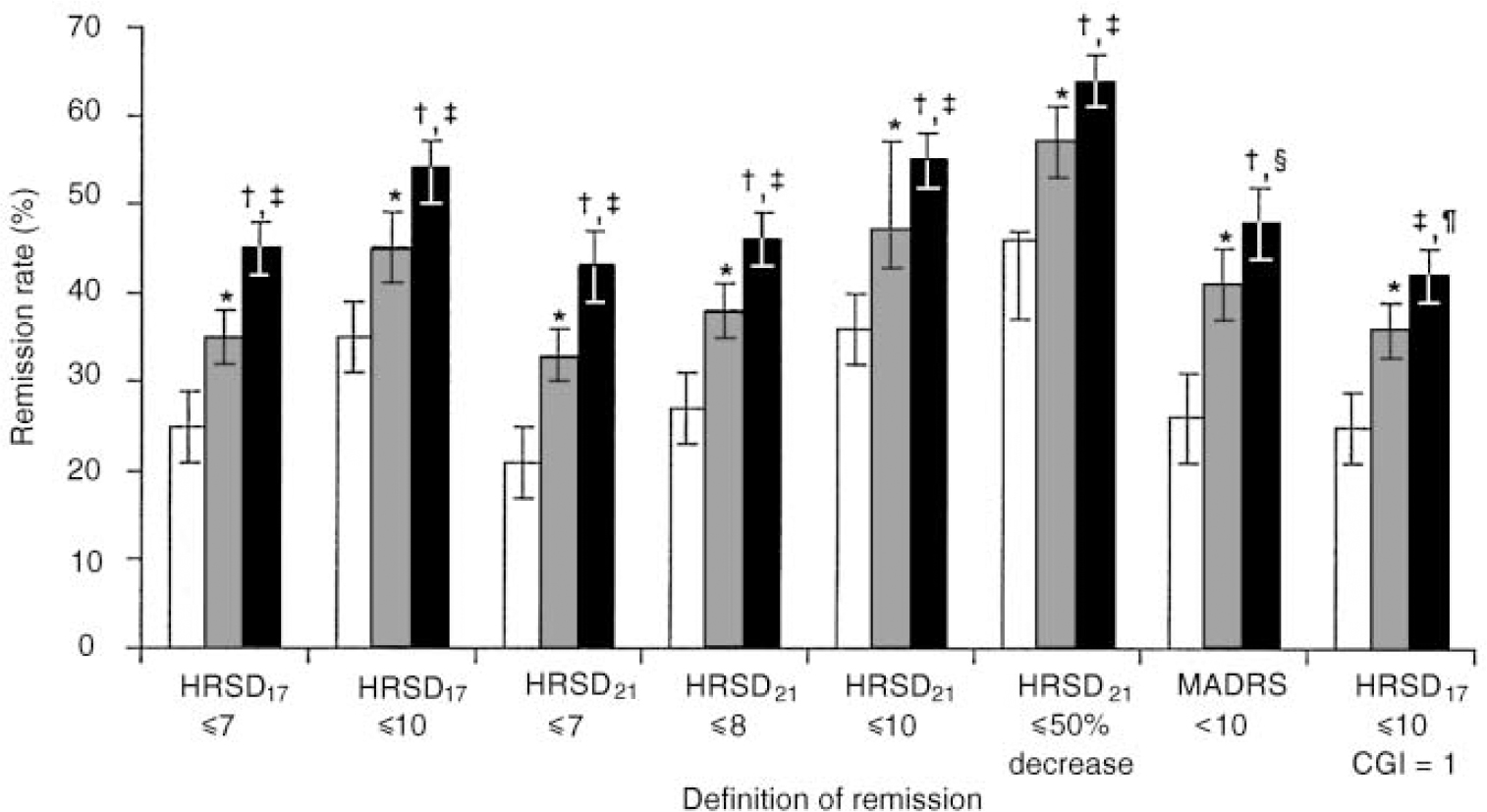
Fig. 3 Final on-therapy remission rates (mean, 95% Cl) with different definitions of remission: *P < 0.001, SSRI (grey bar) v. placebo (white bar); †P < 0.001, venlafaxine (black bar) v. SSRI; ‡P < 0.001, venlafaxine v. placebo; §P=0.023, venlafaxine v. SSRI; ¶ P=0.014, venlafaxine v. SSRI. HRSD, Hamilton Rating Scale for Depression; SSRI, selective serotonin reuptake inhibitor; MADRS, Montgomery—Åsberg Depression Rating Scale; CGI, Clinical Global Impression scale.
In total, 83 (9%) patients were withdrawn from venlafaxine therapy because of side-effects, compared with 57 (7%) SSRI-treated patients and 10 (2%) patients given placebo (Fisher's exact test, P=0.001, venlafaxine v. placebo and SSRI v. placebo; the venlafaxine v. SSRI comparison was not significant, P=0.185). A total of 33/895 (4%) of the venlafaxine-treated patients were withdrawn because of lack of efficacy, compared with 46/769 (6%) of patients given an SSRI and 63/453 (14%) of patients given placebo (Fisher's exact test, P=0.037, venlafaxine v. SSRI; P=0.001, venlafaxine v. placebo; P=0.001, SSRI v. placebo).
DISCUSSION
Are all antidepressants equally effective?
It is often stated that the various different classes of antidepressant medication are equally effective (American Psychiatric Association, 1993; Depression Guideline Panel, 1993). However, the methods used to conduct randomised clinical trials render them relatively insensitive to possible differences between active antidepressants (Reference ThaseThase, 1999). Studies seldom compare groups larger than 120 patients, which does not afford the statistical power to detect modest but still clinically meaningful differences. In addition, multi-site trials may have relatively lower statistical power because of greater patient heterogeneity and lower reliability of diagnoses or dependent measures (Reference ThaseThase, 1999). Moreover, the composition of study groups can have a marked influence on the apparent efficacy of a treatment (Reference Quitkin, Stewart and McGrathQuitkin et al, 1993; Reference Thase, Greenhouse and FrankThase et al, 1997).
Meta-analysis provides useful alternative methods to compare active treatments. For example, meta-analyses comparing tricyclic antidepressants and SSRIs found differences in subgroup comparisons not apparent in qualitative reviews (Reference Anderson and TomensonAnderson & Tomenson, 1994; Reference Edwards and AndersonEdwards & Anderson, 1999). However, because the statistical power of a conventional meta-analysis is determined by the number of studies included, a large number of comparative trials must be available. For comparisons between newer antidepressants, meeting this requirement is often difficult. A second type of meta-analysis, using the data of individual patients participating in a series of related clinical trials, permits powerful comparisons to be made with a much smaller number of studies. Such pooled analyses have been used to document the efficacy of monoamine oxidase inhibitors in the treatment of atypical depression (Reference Quitkin, Stewart and McGrathQuitkin et al, 1993), to examine the association between fluoxetine and suicidality (Reference Beasley, Dornseif and BosomworthBeasley et al, 1991), to examine the effects of venlafaxine treatment on blood pressure (Reference ThaseThase, 1998) and to compare psychotherapy and pharmacotherapy (Reference Thase, Greenhouse and FrankThase et al, 1997; Reference DeRubeis, Gelfand and TangDeRubeis et al, 1999).
The clinical significance of the magnitude of the differences between venlafaxine and the SSRIs warrants comment. In a conventional antidepressant clinical trial, the size of the study groups is such that statistically significant effects parallel relatively large differences in response rates (i.e. 20-25%) that are clearly clinically significant. An analysis of pooled data from an extremely large group of patients, by contrast, would have the statistical power to detect differences in remission rates so small that they would be considered trivial by most (i.e. 3-5%). The difference in remission rates observed in our pooled analysis is roughly halfway between these extremes. Given the high prevalence of depression and the staggering associated illness burden, a 10% advantage in remission rates could have substantial public health implications, particularly if costs and tolerability are comparable. From another perspective, we observed that venlafaxine-treated patients had a 50% greater chance of attaining remission than patients treated with an SSRI. In terms of the number of patients needed to treat to realise a difference, ten patients would need to be treated with venlafaxine in order to obtain one extra case of remission when compared with the SSRIs. When considered together, these various indicators point to a clinically meaningful difference.
Relationships to pharmacological mechanisms
It is proposed that the greater efficacy of venlafaxine is the result of reuptake inhibition of both serotonin and noradrenaline. Of course, reuptake inhibition is not essential to therapeutic action and it is possible that medications that potently and selectively affect either serotonergic or noradrenergic neurotransmission may initiate cascades of intracellular events that ultimately modulate the same changes in gene activity (Reference Duman, Heninger and NestlerDuman et al, 1997). Nevertheless, several previous studies found clomipramine, another potent dual reuptake inhibitor, to have a significant advantage relative to SSRIs (see Reference Anderson and TomensonAnderson & Tomenson, 1994). It appears that relatively higher doses of venlafaxine may be necessary to achieve significant noradrenergic effects, as inferred from in vitro (Reference Muth, Haskins and MoyerMuth et al, 1986; Owens et al, 2000), animal (Reference Redrobe, Bourin and ColombelRedrobe et al, 1998) and human (Reference ThaseThase, 1998; Reference Harvey, Rudolph and PreskornHarvey et al, 2000) studies. Consistent with this, there is a clear dose—response relationship for venlafaxine (Reference Rudolph, Fabre and FeighnerRudolph et al, 1998b ) and patients who fail to benefit from 75 mg/day often respond to higher doses (Reference Dierick, Ravizza and RealiniDierick et al, 1996; Reference Costa e SilvaCosta e Silva, 1998; Reference Diaz-Martinez, Benassinni and OntiverosDiaz-Martinez et al, 1998; Reference Mehtonen, Behnke and SøgaardMehtonen et al, 2000). Therefore, it is likely that the difference in efficacy between venlafaxine and SSRIs is dose dependent. Unfortunately, the flexible dose schedules utilised in five of the studies included in our meta-analysis precluded a valid examination of dose—response relationships. Research using modern molecular biological techniques would help to confirm that the greater antidepressant efficacy of venlafaxine is directly linked to a dual reuptake—inhibitory mechanism of action.
Review of other comparative studies
The most important limitation of a pooled analysis is that the results can be biased by selection of a non-representative group of studies. Our data set included all eight comparative studies conducted by the Wyeth—Ayerst Clinical Research and Development department; no studies were excluded. However, there are at least 12 other studies comparing venlafaxine and SSRIs for treatment of non-psychotic depression. Among these, three recently completed studies (double-blind, placebo- and fluoxetine-controlled trials in out-patients with melancholia, in-patients with melancholia or elderly patients) could not be included because data analyses were not complete. The remaining nine published studies were not included because we did not have access to the original data sets (see Table 4).
Table 4 Summary of intent-to-treat remission rates of nine venlafaxine—SSRI comparative studies of non-psychotic depression not included in pooled analysis

| Study | Setting | Duration (weeks) | Treatment (n) | Dosage (mg/day) | Remission criterion | ITT remission rate (%) |
|---|---|---|---|---|---|---|
| Reference Tylee, Beaumont and BowdenTylee et al, 1997 | PC | 12 | Venlafaxine IR (171) | 75 | MADRS ≤6 | 35 |
| Fluoxetine (170) | 20 | 34 | ||||
| Reference McPartlin, Reynolds and AndersonMcPartlin et al, 1998 | PC | 12 | Venlafaxine XR (183) | 75 | HRSD ≤ 6 | 54 |
| Paroxetine (178) | 20 | 52 | ||||
| Reference Diaz-Martinez, Benassinni and OntiverosDiaz-Martinez et al, 1998 | OP | 8 | Venlafaxine IR (70) | 75-150 | CGI=1 | 41 |
| Fluoxetine (75) | 20-40 | 36 | ||||
| Reference Costa e SilvaCosta e Silva, 19981 | OP | 8 | Venlafaxine IR (196) | 75-150 | CGI=1 | 58 |
| Fluoxetine (186) | 20-40 | 35 | ||||
| HRSD ≤ 7 | 60 | |||||
| Reference Poirier and BoyerPoirier & Boyer, 1999 | OP/IP | 6 | Venlafaxine IR (61) | 75-300 | HRSD < 10 | 37 |
| Paroxetine (62) | 20-40 | 18 | ||||
| Alves for the Venlafaxine Study Group (1999) | OP | 12 | Venlafaxine IR (40) | 75-150 | HRSD ≤ 8 | 30 |
| Fluoxetine (47) | 20-40 | 11 | ||||
| Reference Mehtonen, Behnke and SøgaardMehtonen et al, 2000 | OP | 8 | Venlafaxine IR (75) | 75-150 | HRSD < 10 | 53 |
| Sertraline (72) | 50-100 | 38 | ||||
| Reference Ballús, Quiros and de FloresBallús et al, 2000 | OP | 12 | Venlafaxine IR (41) | 75-150 | HRSD < 8 | 59 |
| Paroxetine (43) | 20-40 | 31 | ||||
| Reference Tzanakaki, Guazzelli and NimatoudisTzanakaki et al, 20001 | IP/PHP | 6 | Venlafaxine IR (55) | 225 | HRSD < 7 | 41 |
| Fluoxetine IR (54) | 60 | 36 | ||||
| CGI=1 | 51 | |||||
| 32 |
It is possible that the inclusion of these additional trials would have affected the findings of the current pooled analysis. We therefore conducted a qualitative review of the nine published studies. Two studies found no evidence of differences in response or remission rates (Reference Tylee, Beaumont and BowdenTylee et al, 1997; Reference McPartlin, Reynolds and AndersonMcPartlin et al, 1998). These studies were conducted in primary care clinics and compared the minimum therapeutic dosages of venlafaxine (75 mg/day) and fluoxetine (20 mg/day) (Reference Tylee, Beaumont and BowdenTylee et al, 1997) or paroxetine (20 mg/day) (Reference McPartlin, Reynolds and AndersonMcPartlin et al, 1998).
Two studies reported non-significant differences (Reference Diaz-Martinez, Benassinni and OntiverosDiaz-Martinez et al, 1998; Alves et al, 1999). Diaz-Martinez et al (Reference Diaz-Martinez, Benassinni and Ontiveros1998) reported that 41% of 70 patients treated with venlafaxine (75-150 mg/day) remitted during an open-label but randomised 8-week trial, as compared with 36% of 75 patients treated with fluoxetine (20-40 mg/day). The difference was 30% (i.e. 50% v. 20%) among those who received either 150 mg/day of venlafaxine (n=18) or 40 mg/day of fluoxetine (n=15). However, this numerically large difference was not statistically significant (P=0.07) in such a small subsample. Alves et al (1999) found a 19% difference (30% v. 11%) in remission rates favouring venlafaxine (75-150 mg/day) over fluoxetine (20-40 mg/day), which again was not statistically significant in a relatively small study (n=87).
Two studies reported inconsistent findings, with significant results favouring venlafaxine over fluoxetine using a global definition of remission but not according to the final HRSD score (see Table 4). Costa e Silva (Reference Costa e Silva1998) observed remission rates of 58% for venlafaxine (75-150 mg/day) and 35% for fluoxetine (20-40 mg/day) using a CGI numeric score of 1 to define remission, although 60% of the patients in each group remitted when an HRSD score of ≤7 was the criterion. Tzanakaki et al (Reference Tzanakaki, Guazzelli and Nimatoudis2000) similarly found that the groups were comparable using an HRSD criterion (<7) but significantly different according to the CGI definition (see Table 4).
The three remaining studies found significant differences favouring venlafaxine; these studies all utilised maximum doses of ≥150 mg/day. Ballús et al (Reference Ballús, Quiros and de Flores2000) observed remission rates of 59% for venlafaxine (75-150 mg/day) and 31% for paroxetine (20-40 mg/day). Mehtonen et al (Reference Mehtonen, Behnke and Søgaard2000), defining remission as a score of <10 on the 21-item version of the HRSD, reported rates of 68% for venlafaxine (75-150 mg/day) and 45% for sertraline (50-100 mg/day) among completers at week 8. Poirier & Boyer (Reference Poirier and Boyer1999) enrolled only patients who had failed to respond to at least two previous trials of antidepressants. About 75% had not responded to a prior course of SSRI therapy. They found a 19% advantage (37% v. 18%) in remission rates in favour of venlafaxine (200-300 mg/day) relative to paroxetine (20-40 mg/day).
Although these studies used various durations of treatment and definitions of remission, two conclusions are evident. First, there is no evidence that venlafaxine is more effective than the SSRIs at minimum therapeutic doses. Second, among the studies that permitted a venlafaxine dosage of ≥150 mg/day, there was a 14.4% average difference (range 5-23%) in remission rates favouring venlafaxine. It appears that the results of our pooled analysis would not have changed if we could have included these studies.
Other limitations
The generalisability of the results of a group of controlled clinical trials, like those of the individual studies, is limited by the exclusion of patients with more complex conditions, such as significant psychiatric and medical comorbidities. Although this lessens the relevance of these results to clinical practice, there is no reason to suspect that this exclusivity favours venlafaxine over the SSRIs. Other potential shortcomings of pooled analyses include problems with the reliability of dependent measures and the possibility that the results may be influenced by the data from one or two particularly large studies. We found significant differences between SSRIs and placebo, however, which indicates that the ‘assay sensitivity’ (Reference LeberLeber, 1991) of the pooled analysis was, at the least, sufficient to overcome measurement error. We also confirmed that the differences were not attributable to any particular study and extended across multiple definitions of remission.
Three more specific limitations can be considered. First, the SSRIs were lumped together as a class. Although there is no evidence that any SSRI is more effective than another, they are not truly interchangeable and some patients respond poorly to one SSRI but well to another (Reference Edwards and AndersonEdwards & Anderson, 1999). In this respect, our pooled analysis included a disproportionate number of patients treated with fluoxetine. The studies listed in Table 4 provide a broader range of comparisons and, in aggregate, yielded similar results. Nevertheless, among the 17 comparative studies included in the pooled analysis or summarised in Table 4, there is only one study each utilising fluvoxamine or sertraline and, to date, there are no studies of citalopram.
Second, all of the studies were short term. It is possible that a longer treatment period could have resulted in comparable remission rates.
Third, none of the studies used in the pooled analysis excluded patients who had failed to respond to other SSRIs. Because several SSRIs were already widely available when these studies were conducted, it is possible that the advantage observed for venlafaxine was delimited to a subgroup of patients who had previously failed trials of other SSRIs (see, for example, Reference Poirier and BoyerPoirier & Boyer, 1999).
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Patients treated with venlafaxine had a 10% greater chance of remission than those treated with SSRIs (45% v. 35%).
-
▪ Onset of remission occurred 1-2 weeks earlier for venlafaxine-treated patients.
-
▪ Doses of ≥ 150 mg/day venlafaxine may be necessary to maximise the likelihood of remission.
LIMITATIONS
-
▪ Results of meta-analysis may be affected by the quality of the individual studies.
-
▪ Generalisability is limited by the exclusivity of clinical trial enrolment.
-
▪ There is not a sufficient number of studies to compare venlafaxine with specific SSRIs other than fluoxetine.












eLetters
No eLetters have been published for this article.