Epidemiologists think that everyone should be interested in how many people have which mental disorders, how disabled they are by them and what services they use and want, but such analyses usually leave health planners and clinicians unimpressed. Health planners want clear directions to improve health gains within the present budget, and clinicians want clear directions to improve the outcomes of individual patients using their present resources. Both would welcome increased budgets. Coverage – the proportion of the population in treatment – appears to be independent of the amount of money that developed countries spend on health, so more money may not be the obvious answer (Reference Andrews, Issakidis and CarterAndrews et al, 2001). Doing what is right need not necessarily cost more, although at times the cost can be prohibitive (Reference Marshall and RouseMarshall & Rouse, 2002). In this paper we build upon published data from the Australian low prevalence survey that answered the ‘how many’, ‘how disabled’ and ‘what services’ questions (Reference Jablensky, McGrath and HerrmanJablensky et al, 2000). We use these results to calculate the burden of schizophrenia currently being averted by services in Australia, the amount that could be averted given optimal treatment and whether such evidence-based treatments could be afforded by the present health service budgets. We cost direct treatment services, calculate the likely reduction in disability burden and calculate the cost-effectiveness of current and optimal treatment (Reference Andrews, Sanderson and CorryAndrews et al, 2000). We place the findings within the World Health Organization method for setting priorities in health research and development (see Fig. 1). We describe here the general method and give the method and results with respect to schizophrenia.
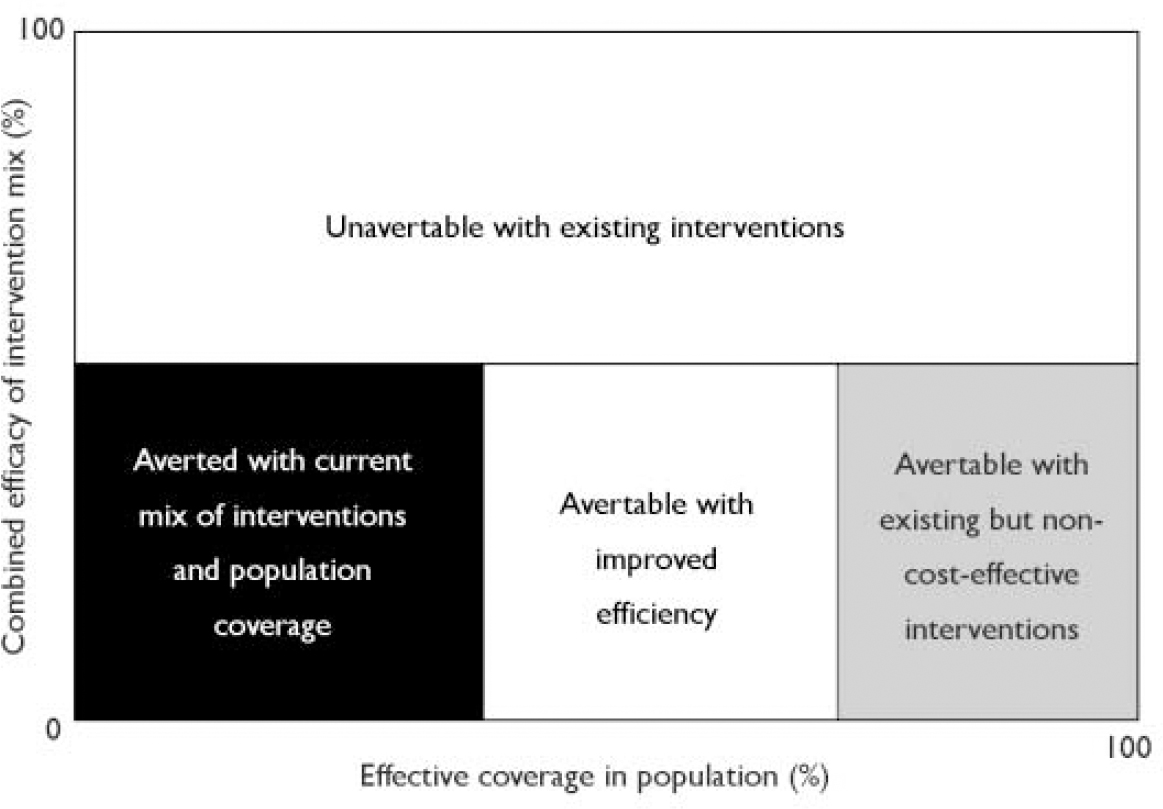
Fig. 1 Relative shares of the burden of disease of a given disorder that can and cannot be averted with existing tools, adapted with permission from the World Health Organization model for analysing the burden of a health problem to identify research needs (Ad Hoc Committee, 1996: p. 7).
METHOD
General method
The model for this investigation is displayed in Fig. 2 and the assumptions of the analysis are numbered in Table 1 and referred to throughout the text by reference to these numbers in parentheses. A 1-year time horizon was used to estimate burden lost, burden averted with interventions and costs, using the reference year of mid-1997 to mid-1998 (Assumption 1). This provides a snapshot of the costs and outcomes over a year for current treatment and what could be achieved in a year with wider implementation of evidence-based interventions (referred to as optimal treatment). The burden of a disease can be estimated in disability-adjusted life-years (DALYs) lost (Assumption 2). A DALY comprises years of life lost owing to premature death from the disorder (YLL) plus years lived with the disorder (YLD), weighted by the disability weighting associated with the disorder.
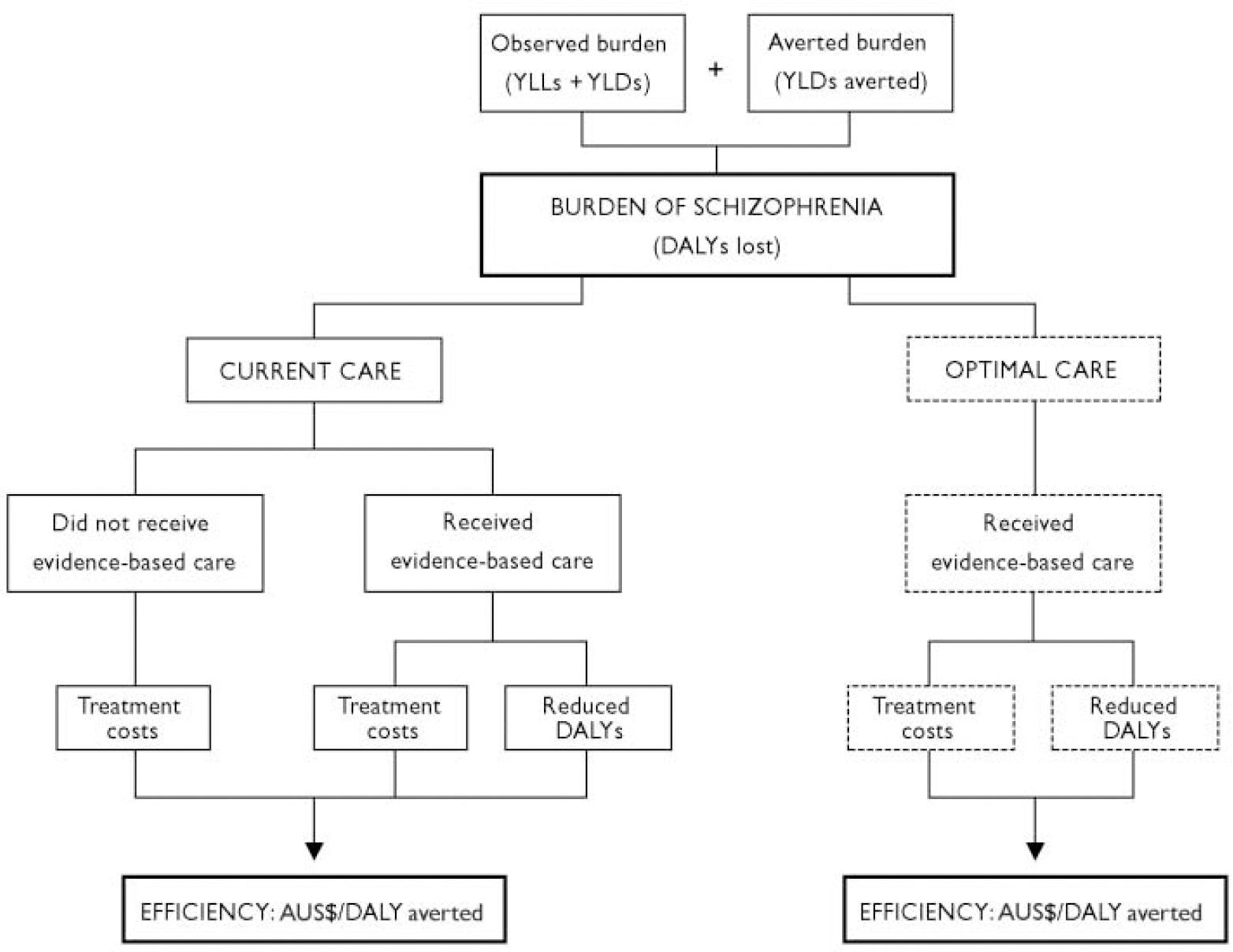
Fig. 2 Inputs required to model the efficiency of current and optimal care in reducing the burden of schizophrenia. YLLs, years of life lost owing to premature death from the disorder; YLDs, years lived with the disorder; DALYs, disability-adjusted life-years.
Table 1 Assumptions of the analysis and the corresponding evidence

| Assumption | Evidence |
|---|---|
| (1) A 1-year time horizon was used to estimate burden lost, burden averted with interventions and costs | This project was examining alternative uses of the total 1-year expenditure on mental-health-related treatment, so a 1-year time frame was appropriate. Also, efficacy was estimated from randomised controlled trials, which rarely measure outcome beyond 1 year, and it is recommended that short and longer time horizons be modelled separately when the analysis must go beyond the time frame of the primary data (Reference Gold, Siegel and RussellGold et al, 1996) |
| (2) Individual health benefits can be reflected in population estimates of disability-adjusted life-years (DALYs) averted, adjusted for time spent symptomatic | Population health as measured by DALYs is an aggregation of individual health, as DALYs is the loss of healthy years owing to premature mortality plus prevalence weighted by severity of disability in individuals |
| (3) The true burden of disease is the burden evident in a population plus the burden averted by current interventions | Measured burden will be ameliorated by the efficacy of existing services, so burden in the absence of services can be estimated by adding measured burden to burden currently averted with existing services |
| (4) Burden, in DALYs lost, can be attributed to the people who identified a principal complaint in the previous 12 months, adjusted for time spent symptomatic | Principal complaint choice allows examination of the relationship between disability and disorder in the presence of comorbidity (Reference Andrews, Slade and IssakidisAndrews et al, 2002) |
| (5) Evidence-based medicine is indicated by self-reported clinician treatment with an effective intervention | Randomised controlled trials support medication and psychological interventions as efficacious for most mental disorders (Reference Nathan and GormanNathan & Gorman, 1996) |
| (6) The degree of change resulting from treatment in effect size units in clinical trials indicates the degree of change in disability weightings used in years lived with disability (YLD) calculations | The relationship between preferences and effect size change was defined for symptoms and disability, whereas effect sizes from meta-analyses predominantly summarise change in symptoms. In a study of schizophrenia symptoms, greater severity elicited less favourable preference values (Chouniard & Albright, 1997) |
| (7) The effect size captures both changes in severity and duration of illness used in YLD calculations | The effect size is a standardised mean difference, and summarises the overall benefit to those who improved and remitted and to those who improved but not enough to remit. An overall effect size thus implicitly includes the benefit of remitted cases, which is equivalent to a reduced duration |
| (8) The comparator is no treatment (i.e. natural history). Natural history can be estimated from waiting-list control studies | Waiting list is a proxy for natural history (Reference Kirsch and SapirsteinKirsch & Sapirstein, 1998) |
| (9) Service use reported by individuals in surveys is applicable to the whole population and sufficiently accurate for bottom-up costing | Self-reported service use from epidemiological surveys is reasonably consistent with other sources of service use, albeit slightly underestimated (Reference Manderscheid, Rae and NarrowManderscheid et al, 1993) |
| (10) Service use can be attributed to the principal complaint during the previous year | This is essential to allow bottom-up costing as each unit of service is only counted once, attributed to a single disorder |
| (11) Efficacy reflects effectiveness under certain conditions | Efficacy from randomised controlled trials includes those who drop out of trials and those who do not comply if an intent-to-treat analysis is used. In addition, treatment resistance is modelled for a proportion of cases (see Assumption 13), and thus in this study the actual magnitude of effect applied at a group level is closer to effectiveness than efficacy |
| (12) It is reasonable to operationalise detailed treatment regimes from clinical practice guidelines informed by the published literature and expert opinion | Clinical practice guidelines summarise research and clinical expertise on optimal care for a disorder and provide the best source for defining optimal care |
| (13) A proportion of patients will be treatment resistant | Some patients do not improve with any form of intervention (Reference Conley and KellyConley & Kelly, 2001) and are attributed no health benefit. These patients incur costs only for maintenance treatment |
In mental disorders, death by suicide or increased physical morbidity are rarely attributed to the underlying mental disorder. In the Australian Burden of Disease study only 2% of the burden of schizophrenia was attributed to YLLs, despite the reduced life expectancy and the suicide rate. In this series of papers we will mention the YLL but develop models using the more important and more easily available YLD data. The true burden of a disorder, defined as burden in the absence of treatment, is calculated from the burden observed in the population under study plus the burden presently averted by the current population coverage and mix of interventions (Assumption 3). The observed burden was calculated from the prevalence of each disorder (identified as a current principal complaint) multiplied by the disability weighting for that disorder, to give the YLD (Assumption 4). Consistent with a previous analysis (Reference Melse, Essink-Bot and KramersMelse et al, 2000), age weighting and discounting were not applied because the former is controversial and the latter was inappropriate given the cross-sectional prevalence perspective of the study.
We identified the YLDs averted by the current mix of services from the proportion of prevalent cases deemed to have received an effective treatment in the past year (Assumption 5). Calculating the YLDs averted by effective treatment is a new field. The primary outcome in mental disorders is measured as changes in symptoms, but improvements in functioning and quality of life are increasingly included (Reference Smith, Manderscheid and FlynnSmith et al, 1997). These are most appropriately represented as changes in the disability weighting in the YLD formula, estimated from the effect sizes in efficacy studies (Reference Andrews, Sanderson and CorryAndrews et al, 2000) (Assumptions 6 and 7). The YLD averted by current treatment added to the observed burden gives baseline burden in the absence of treatment. This serves as a comparator in the analysis (Assumption 8) and provides the baseline for calculating the percentage of burden averted.
The respondents to the surveys listed the services used and treatments received for a mental health problem during the previous 12 months (Reference Andrews, Issakidis and CarterAndrews et al, 2001) (Assumption 9). Unit costs for each service or procedure were obtained from published sources and expressed in the values of the reference year (1997–1998 constant Australian dollars) using the consumer price index health deflators. The cost of services used in the previous year was calculated for each person with a principal complaint of a mental disorder (Assumption 10). The average 12-month cost per case of each disorder was calculated and, when divided by the number of YLDs averted, gave a simple cost-effectiveness ratio in dollars per YLD averted for each disorder.
Next we calculated the proportion of the burden that could be averted with improved efficiency. We assumed that coverage remained at the present levels, that clinicians only used evidence-based treatments and that compliance with treatment paralleled that seen in efficacy studies (Assumption 11), and again calculated the cost per case and cost per YLD of optimal treatment. Accepting that there is unlikely to be a radical increase in the mental health service budgets, we ask whether the money spent on optimal treatment would be more or less than that spent on the current mix of treatments. If optimal treatment at the current level of coverage leaves money unspent, we ask if other, presently non-cost-effective interventions should be purchased or whether the money would be best spent on increased coverage (see Fig. 1).
These calculations were informed by a sensitivity analysis conducted with the @RISK version 4 software (Newfield, NY: Palisade Corporation) for Microsoft Excel, which uses a Monte-Carlo simulation approach to provide 95% confidence intervals around YLDs averted, total cost of treatment and cost per YLD averted. A multivariate stepwise linear regression was also conducted for each of the above three estimates, with this estimate as the dependent variable and the individual cost and outcome units of data as the independent variables. This analysis identifies the most important contributors to variance in each parameter. In addition, univariate sensitivity analyses were conducted on variables that were defined by the investigators in consultation with experts, with these variables varied individually to determine their impact on the above estimates. Finally, we calculated the effect of optimal treatment on burden averted, given total coverage and concordance, and expressed this as the proportion of burden theoretically avertable with existing knowledge. The remaining burden is that unavertable with existing knowledge and interventions (shown by the white box in Fig. 1) and is an indication of the need for investment in research and development.
Specific method for schizophrenia
Burden of disease
The YLD from schizophrenia was calculated from the prevalence of the disorder multiplied by the disability weighting. The 1-month prevalence was taken from Jablensky et al (Reference Jablensky, McGrath and Herrman2000), a catchment area study of low-prevalence psychotic disorders (n=980). This group was divided into severity categories based on longitudinal course of illness characteristics (Reference Jablensky, McGrath and HerrmanJablensky et al, 2000), with the proportion of the total group in each category as follows: new incident cases (2%); people who experienced complete or partial remission between episodes, defined as no psychotic symptoms but residual anxiety and depression (30%); people who experienced negative symptoms between episodes (23%); and people who experienced a continuous level of psychotic symptomatology without resolution between episodes (45%). Apart from this last group, it was estimated that 23% of a person's time would be spent in a psychotic episode (Reference Wiersma, Nienhuis and SlooffWiersma et al, 1998). A disability weighting of 0.82 for acute psychosis was used (Reference Sanderson and AndrewsSanderson & Andrews, 2001), whereas for time spent in remission with residual anxiety/depressive symptoms the disability weighting of 0.34 for moderate depression was applied (Reference Sanderson and AndrewsSanderson & Andrews, 2001) and for the time in remission with negative symptoms a milder weighting of 0.46 for schizophrenia was applied (taking the average of a milder and more severe schizophrenia weighting; Stouthardt et al, 1997). These data provided a composite disability weighting of 0.638.
Current and optimal treatment
The type and amount of services currently being received was obtained from Jablensky et al (Reference Jablensky, McGrath and Herrman2000) and from additional data supplied by those authors. Optimal treatment was defined by recommendations in the clinical practice guideline literature, primarily the PORT study recommendations (Reference Lehman and SteinwachsLehman & Steinwachs, 1998) (Assumption 12), which continues to be an industry standard (Reference Milner and ValensteinMilner & Valenstein, 2002). Interventions of interest were those that improve clinical and functional outcomes, because these are directly relevant for mapping to changes in the disability weighting used in calculating the YLDs averted. These recommendations were operationalised into detailed treatment regimes on the basis of published literature and expert opinion (Assumption 12). Because there were numerous possibilities for the distribution of the prevalence cohort across different treatment providers and interventions, a range of values for these parameters was incorporated in the sensitivity analysis (as described above). In both current and optimal scenarios the interventions were linked to meta-analyses to estimate their efficacy and hence the change in disability weighting, with analyses chosen for methodological rigour and ability to code overall effect sizes of treatment benefit (Reference Skelton, Pepe and PineoSkelton et al, 1995; Reference Mojtabai, Nicholson and CarpenterMojtabai et al, 1998; Reference Leucht, Pitschel-Walz and AbrahamLeucht et al, 1999; Reference Jones, Cormac and MotaJones et al, 2000).
The benefits of medication plus psychosocial interventions were considered additive (Reference Mojtabai, Nicholson and CarpenterMojtabai et al, 1998), whereas if a person received more than one psychosocial intervention they were attributed the benefit of the treatment with the largest effect size. A transfer factor of 0.181 was derived and used to transform effect size superiority over placebo due to treatment into preference weighting change due to treatment (Reference Andrews, Sanderson and CorryAndrews et al, 2000). Because the specific placebo effect is negligible in schizophrenia (Quality Assurance Project, 1984), the improvement observed in placebo groups represents natural history and/or regression to the mean, so these effect sizes represent the benefit in comparison with natural history and are thus consistent with the comparator of no treatment in the analysis (Assumption 8). Some patients remain asymptomatic despite sequential treatment with different drug categories, referred to as treatment resistance (Assumption 13). Treatment resistance was modelled at 20% (Reference Conley and KellyConley & Kelly, 2001). It should be noted that effect sizes for specific interventions and not mode of delivery were used, because treatment outcome is largely dependent on interventions received rather than the system of care under which it is delivered (Reference Thornicroft, Becker and HollowayThornicroft et al, 1999). Systems of care such as case management are included in the hypothetical model of optimal care for the present study because they are important for coordination of efficacious services (Reference Lehman and SteinwachsLehman & Steinwachs, 1998), but costs rather than outcome have been attributed to these service systems. There is as yet no evidence for the prevention of schizophrenia (Reference WarnerWarner, 2001), so overall prevalence remained unchanged.
Cost of services used
The perspective of this study was ‘government’ or funder costs and it does not take account of any ‘out of pocket’ or indirect costs. These additional calculations are available from Carr et al (Reference Carr, Neil and Halpin2003). Costs were calculated for the reference year and unit costs obtained from other years were converted to 1997–1998 costs using the health component of the consumer price index. Costs are for 12 months of treatment. Two additional calculations were included for the cost of current care for schizophrenia to ensure consistency with other disorder analyses, because these other analyses were based on data from the Australian National Survey of Mental Health and Well-Being (Reference Andrews, Slade and IssakidisAndrews et al, 2002). Using responses from screener-positive cases on a psychosis screener used in the National Survey, we estimated the total treatment costs for other contacts for a mental health problem (including radiologists, pathologists, general medical specialists and other counsellors). We also adjusted the number of general practitioner contacts from the low prevalence survey (Reference Jablensky, McGrath and HerrmanJablensky et al, 2000) (which were not specifically mental-health-related contacts) by the proportion of all such contacts that were mental health related in the National Survey (35.6%), such that we were costing only mental-health-related general practitioner contacts.
RESULTS
Burden of disease
Twenty-four deaths were attributed to schizophrenia in the reference year 1997, with three-quarters of the deaths in the 65-year or older age group. The total YLLs lost from these deaths was 402 and, as mentioned earlier, these underestimates were not used. The 1-month prevalence of schizophrenia was 0.29% (39 048 individuals in the Australian population) and the disability weighting was 0.638. The total YLD is the product of these two numbers: 24 913.
Description and outcome of current and optimal treatment
Current treatment
The Jablensky et al (Reference Jablensky, McGrath and Herrman2000) survey was a catchment area study that identified all persons known to medical and social services. The pattern of contacts across various health sector providers and the interventions received are presented in Tables 2 and 3. Nearly all persons were being prescribed an antipsychotic medication (haloperidol or other typical antipsychotic, 51%; atypical antipsychotic, 25%, clozapine, 12%). A much smaller proportion was receiving a psychosocial intervention. The proportions for social skills training and psychological therapies were discounted by 50% in the efficacy calculation, because it was considered unlikely that the full proportion were receiving an evidence-based version of this intervention (Reference Carr, Neil and HalpinCarr et al, 2003). The disability weighting change attributed to these pharmaceutical and psychosocial interventions was derived from the weighted effect size multiplied by the transfer factor of 0.181, providing an overall disability weighting improvement of 0.121. This drops to 0.097 when the 20% treatment-resistant group is included. This produced a burden averted of 3774 YLDs (see Table 3). Thus, the adjusted disability weighting for the null position of no treatment in the Australian population was 0.638+0.097 or 0.734, and the untreated or baseline disability burden of schizophrenia was thus 28 671 YLDs. These data show that only 13.2% of the disability burden of schizophrenia is averted by current services.
Table 2 Description of current and optimal mental-health-related treatment for schizophrenia and schizoaffective disorder in Australia

| n | Proportion treated with each type of care | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mental health sector | Pharmaceuticals | General health sector: GP3 (%) | ||||||||
| Acute in-patient admission1 (%) | Longer-stay in-patient admission1 (%) | Psychiatrist (%) | Psychologist (%) | Mental health team2 (%) | Typical antipsychotic (%) | Atypical antipsychotic (%) | Clozapine (%) | |||
| Current treatment | 39 048 | 36 | 12 | 21 | 4 | 57 | 51 | 25 | 12 | 85 |
| Optimal treatment | 39 048 | 45 | 6 | 16 | 10 | 79 | 34 | 55 | 11 | 16 |
| New incident cases | 781 | 30 | 0 | 50 | 50 | 50 | 0 | 100 | 0 | 50 |
| Complete/partial remission | 11 871 | 36 | 0 | 50 | 30 | 50 | 0 | 100 | 0 | 50 |
| Negative syndrome | 8825 | 36 | 0 | 0 | 0 | 100 | 30 | 60 | 10 | 0 |
| Continuously symptomatic | 17 571 | 57 | 13 | 0 | 0 | 89 | 60 | 20 | 20 | 0 |
Table 3 Comparative efficacy of current and optimal treatment strategies for schizophrenia and schizoaffective disorder

| Pharmacological treatment | Psychosocial treatment1 | Total improvement2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Typical | Atypical | Clozapine | Family therapy | Social skills training | Cognitive-behavioural therapy | Disability weighting change | YLDs averted | |
| Effective size | 0.47 | 0.50 | 1.17 | 0.56 | 0.44 | 0.76 | ||
| Current treatment (%)3 | 51 | 25 | 12 | - | 16 | 36 | 0.121 | 3774 |
| Optimal treatment (%)3 | 34 | 55 | 11 | 37 | 27 | 47 | 0.159 | 6217 |
| New incident cases | 0 | 100 | 0 | 80 | 0 | 100 | 0.227 | 177 |
| Complete/partial | 0 | 100 | 0 | 50 | 0 | 80 | 0.220 | 2611 |
| remission | ||||||||
| Negative syndrome | 30 | 60 | 10 | 50 | 80 | 50 | 0.213 | 1881 |
| Continuously symptomatic | 60 | 20 | 20 | 20 | 20 | 20 | 0.159 | 1548 |
Optimal treatment
We operationalised optimal care as described by the PORT study (Reference Lehman and SteinwachsLehman & Steinwachs, 1998) for the severity groups described earlier. The disability weighting changes were derived from the effect sizes associated with the treatments used, exactly as was done for current care (see Table 3). The groups were new incident cases (disability weighting change=0.227), complete or partial remission (disability weighting change=0.220), remission with negative syndrome (disability weighting change=0.213) and continuous psychotic symptoms (disability weighting change= 0.159). This provides an overall disability weighting change of 0.159, providing 6217 YLDs averted or 21.7% of baseline YLDs.
Cost and efficiency of current and optimal treatment
Current treatment
The unit costs of treatment are provided in Table 4, which were combined with the data on service contact in Table 3 to provide the total cost. The average cost of a person with schizophrenia being treated for 1 year was AUS$18 949 in 1997–1998 and the total direct governmental cost of schizophrenia was the cost per case multiplied by the number of cases, or AUS$740 million. This money averted 3774 YLD, giving a cost-effectiveness ratio of AUS$196 070 per YLD gained.
Table 4 Cost of current and optimal mental-health-related treatment for schizophrenia and schizoaffective disorder in Australia

| Cost of each type of care for 12 months in 1997-1998 (AUS$) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mental health sector | Pharmaceuticals | Aggregated costs | |||||||||
| Acute in-patient admission | Longer-stay in-patient admission | Psychiatrist | Psychologist | Mental health team | Typical anti-psychotic1 | Atypical anti-psychotic2 | Clozapine | General health sector: GP | Sum of all costs | Cost per treated case | |
| Unit cost (AUS$) | 348.05 per | 272.43 per | 119.33 per | 80.00 per | 99.44 per | 0.51 per | 5.79 per | 10.87 | 41.38 per | ||
| bed-day | bed-day | contact | contact | treatment day | day | day | per day | contact | |||
| Current treatment costs3 | 226.0 | 409.5 | 13.1 | 3.1 | 36.3 | 3.8 | 20.4 | 20.3 | 5.5 | 740.0 | 18 949 |
| (AUS$ million) (n=39 048) | |||||||||||
| Optimal treatment costs4 | 142.2 | 204.7 | 9.1 | 1.9 | 240.3 | 2.5 | 44.9 | 17.5 | 3.1 | 668.2 | 17 113 |
| (AUS$ million) (n=39 048) | |||||||||||
Optimal treatment
As for current care, the unit cost data in Table 4 were combined with service use data in Table 2 to estimate the total cost of treatment. The average cost of a person with schizophrenia being treated for 1 year (AUS$17 113) was very similar to that of current care, providing a total population cost of AUS$668 million. Bed-day costs account for half of this expenditure, down from 85% with current care. Optimal treatment models shorter overall bed-days, especially for longer-stay beds with the advent of clozapine, and a greater use of community-based services. For this expenditure a higher number of YLDs were averted (6217), giving a cost-effectiveness ratio of AUS$107 482 per YLD gained.
Comparative efficiency
When current care and optimal care are compared, the number of YLDs averted increases by two-thirds but the cost remains stable, so the AUS$/YLD averted is reduced and efficiency improves. The proportion of burden averted changes similarly, rising from 13% to 22% (Table 5). The 95% confidence intervals are also presented in Table 5, indicating significant variation according to the information used to estimate costs and outcomes. As part of the sensitivity analysis, linear regression analyses showed that the most important variables that determined variation in YLDs averted were the cognitive–behavioural therapy and haloperidol effect sizes, and the transfer factor to convert these to disability weighting changes. Similarly for optimal care, the most important predictors were the cognitive–behavioural therapy and risperidone effect sizes and the transfer factor. Variation in total costs for both optimal and current care were driven by the unit costs, with the largest variance estimates around case manager contacts and bed-days. When these two estimates were put together to provide cost per YLD averted, the most important predictors in variation were the acute and non-acute bed-day unit costs and the cognitive–behavioural therapy effect size for current care, and standard case manager unit cost, intensive case management number of contacts and cognitive–behavioural therapy effect size for optimal care.
Table 5 Comparative efficiency, in cost per year lived with disability (YLD) averted, of current and optimal treatment for schizophrenia and schizoaffective disorder

| n | Efficacy (YLDs averted) | Cost per treated case (AUS$) | Total cost of treatment (AUS$ million) | Efficiency (AUS$ per YLD averted) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Point estimate | 95% CI | % burden averted | Point estimate | Point estimate | 95% CI | Point estimate | 95% CI | ||
| Current treatment | 39 048 | 3774 | 2908-4691 | 13 | 18 949 | 740.0 | 484.7-1020.2 | 196 070 | 123 827-297 516 |
| Optimal treatment | 39 048 | 6217 | 4326-8382 | 22 | 17 113 | 668.2 | 408.5-1133.3 | 107 482 | 59 714-205 418 |
The univariate sensitivity analyses showed that although some investigator assumptions did have an impact on the cost per YLD, the overall conclusions were not affected. For current care, we estimated that 50% of those receiving psychosocial interventions received an evidence-based version of this treatment. If we take a more optimistic view and increase this to 80%, the cost per YLD reduces by 13% to AUS$170 297. If this is decreased to the more realistic scenario of 20%, the cost per DALY increases by 18% to AUS$231 473. Both estimates are still large and substantially less efficient than the point estimate for optimal care. Similarly, the conclusions for optimal care are not changed if we double the proportion of people on clozapine (cost-effectiveness decreases by 2% to AUS$109 098), or increase total bed-days by 50% (efficiency declines by 11% to AUS$118 917). If we reduce by 50% the proportion of people with a case manager as their primary clinician, and assign these people to psychiatrist-managed care, the efficiency improves by 17% to AUS$89 015. A more optimistic value of 10% of prevalent cases considered treatment resistant improves efficiency by 11% for current treatment and 9% for optimal treatment. If a more pessimistic scenario of 30% treatment resistance is modelled, efficiency is reduced by 14% for current treatment and 11% for optimal treatment.
The low prevalence study (Reference Jablensky, McGrath and HerrmanJablensky et al, 2000) identified patients known to services and it is not surprising that all were in receipt of some treatment. If they constitute the vast majority of people with schizophrenia, then the theoretical upper limit of the burden of schizophrenia that is able to be averted by current knowledge, even given perfect coverage, competence and compliance, is less than one-third of the burden, presuming that 90% of cases are already in treatment.
DISCUSSION
Reprise
The cost-effectiveness of current treatment of schizophrenia in Australia was just under AUS$200 000 per YLD (or DALY) gained. If the PORT study guidelines (Reference Lehman and SteinwachsLehman & Steinwachs, 1998) were operationalised as optimal care then, at the same level of coverage as in current treatment and with compliance similar to that in efficacy trials, the total cost to the health system remains stable but the health gain increases by 65%. Optimal treatment is estimated to cost AUS$107 000 per YLD gained. Evidence-based medicine is affordable. Current treatment was estimated to avert 13% of the burden of schizophrenia in a year, and optimal care averts an additional 9%. There are no other, presently affordable, intervention strategies that could be expected to lower the burden further. Using the model depicted in Fig. 1, we are left with the uncomfortable realisation that the majority of the burden of schizophrenia is simply unavertable in the light of current knowledge.
Threats to validity
This is a modelling study and many assumptions have been made. The assumptions and supporting references are listed in Table 1. The prevalence and coverage in the Jablensky et al (Reference Jablensky, McGrath and Herrman2000) data came from what was an urban-based treated prevalence study. Because 85% of Australians live in urban regions and over 90% of the population are treated in urban regions, the results should be considered representative. If the prevalence had been corrected for the people in the community not known to services (Reference Link, Dohrenwend, Dohrenwend, Dohrenwend and GouldLink & Dohrenwend, 1980), then the prevalence would rise to 0.35% and the coverage would drop to about 80%. The latter is closer to the 60% coverage calculated from the Dutch NEMESIS survey (Reference Bijl, Ravelli and van ZessenBijl et al, 1998) once they added patients in institutions to those identified in the community survey.
People with schizophrenia have a reduced life expectancy (Reference Andrews, Hall and GoldsteinAndrews et al, 1985) yet YLLs are poorly represented by the data, especially those due to suicide. Thus the burden of the disease will be greater than that estimated. Changes in DALYs due to intervention are calculated from changes in YLDs but we are unaware of any data showing that current or optimal treatment prolongs life. The estimate of the burden averted by treatment may not be low but the proportion of burden averted may be an overestimate. The method for estimating change in disability weighting was developed for this project. When compared with the few prospective studies that have measured both changes in mental health status and health state preference values, it gives comparable results (Chouniard & Albright, 1997; Reference Andrews, Sanderson and CorryAndrews et al, 2000). From the evidence to date it is thus likely to be appropriate even if the transfer factor between effect sizes and disability weighting change is updated by future research. Modelling atypical antipsychotics in favour of typical antipsychotics is controversial (Reference Geddes, Freemantle and HarrisonGeddes et al, 2000). If half of those modelled to receive an atypical antipsychotic under optimal treatment are moved to a typical antipsychotic (haloperidol), then efficiency is reduced by 6% to AUS$114 440 per YLD averted. Similarly, the evidence for the efficacy of social skills training has been questioned recently (Reference Pilling, Bebbington and KuipersPilling et al, 2002). This intervention had the smallest effect size of the psychological strategies included and was only modelled to the two most severe groups. These two groups were also receiving one of the other recommended psychological therapies (family therapy or cognitive–behavioural therapy), so removing social skills training from the analysis does not alter the results.
The prevalence, service use and unit cost data are from Australia but because Australia has fewer psychiatric beds than other established market economies (World Health Organization, 2001) the current treatment is unlikely to be more expensive than in other developed countries. Nevertheless, there will be country-specific differences in bed-days and pharmaceutical costs. The finding that optimal care is no more expensive but twice as efficient is likely to be transferable. The costs of implementing evidence-based treatment have not been included and the magnitude of the added benefits in relation to these initial and ongoing costs may not always be favourable (Reference Mason, Freemantle and NazarethMason et al, 2001). The finding that only a modest degree of the burden of schizophrenia and schizoaffective psychosis is currently averted is consistent with clinical experience (Reference McGlashan and JohannessenMcGlashan & Johannessen, 1996); the finding that optimal care would leave the majority of the burden unaffected is of serious concern.
Relation to other work
This work began with three questions. What proportion of the burden of each mental disorder is being averted by current treatment? What proportion could be averted by optimal treatment? And, if optimal treatment is superior, is it affordable? The answer in schizophrenia is clear: optimal treatment costs no more and substantially increases the health gain. This project has covered all the common mental disorders (affective, anxiety and substance use disorders), the results for these disorders are not dissimilar (publication forthcoming). Whether results for a physical disease with similar impact would correspond is not known. It would be interesting to evaluate the cost-effectiveness of treatment for a disease such as rheumatoid arthritis.
Implications
The cost of treatment for schizophrenia is high. It is about AUS$20 000 per year on average and AUS$30 000 per year for the substantial minority who have continuous psychotic symptoms. This annual expenditure is simply unaffordable by patients or their families, especially as treatment is not sufficiently successful to return most sufferers to the labour force. Even targeted interventions such as supported employment have only moderate efficacy (Reference Crowther, Marshall and BondCrowther et al, 2001). It is not surprising therefore that treatment of schizophrenia in developed countries is largely supported by public sector services. In Musgrove's terms (Reference MusgroveMusgrove, 1999), public expenditure is justified on many grounds: catastrophic annual cost, poverty of the sufferers, externality of danger that some patients pose to society and the simple rule of rescue. The public is afraid of psychosis and demands that people with schizophrenia be cared for. The public did so 50 years ago when there was no effective treatment, an attitude that illustrates that cost-effectiveness is not the only determinant of the provision of care.
Efficiency, measured as cost-effectiveness, is low. The affordable price in AUS$/DALY for health care in the public sector is not absolute, but does tend to be around the average annual wage for a country. The average weekly wage in Australia in 1997–1998 was AUS$591.40 (Australian Bureau of Statistics, 1998), which is equivalent to AUS$30 753 per annum. We estimated that current treatment for schizophrenia costs some AUS$200 000 per DALY, which is well above the affordable price. Even optimal care at half this figure is well above the affordable price. It is clear that current care should move towards the pattern of optimal care, that is, towards evidence-based medicine. The changes are not complex: reduced bed-days, particularly longer-stay, would reduce the cost; and increased use of atypical antipsychotic drugs and psychological treatments would increase the cost but also improve the effectiveness. But schizophrenia is complex and, because of the acuteness of many presentations, it is simply not possible always to carry out what is optimal. Emergencies, for example, are costly. It is likely therefore that the attainable cost-effectiveness in practice will lie somewhere between the current and optimal figures.
Faced with a costly and only modestly effective treatment, it is usual to say that more research is required. The question is whether it is reasonable to invest in an enlarged research programme. The World Health Organization five-step model for investing in health research and development (Ad Hoc Committee, 1996) suggests the following steps when thinking about this problem.
-
(a) Calculate the burden of a disease.
-
(b) Identify the reason why the disease burden persists.
-
(c) Judge the adequacy of the current knowledge base.
-
(d) Assess the promise of the research and development effort in terms of the probability of a successful development of a cost-effective intervention.
-
(e) Assess the magnitude of the current effort and the additional cost of developing a new intervention.
Schizophrenia has a high burden, being ranked 13th of all diseases in established market economies (Ad Hoc Committee, 1996: Table A1.3). We conclude that only 13% of the burden is presently being averted, in part because we do not make the best use of existing cost-effective interventions. Nevertheless, three-quarters of the burden seems unavertable with existing interventions, and new ones are required. The knowledge base does not yet identify the direction from which new interventions could be developed. Strategic research is necessary to strengthen that knowledge base. Until that occurs, one could not estimate the promise or cost of a research and development effort and it would be difficult to meet the criteria for deciding that the research would be a wise investment of resources (see Fig. 1). This is a serious conclusion.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ ▪ Schizophrenia is expensive to treat and current treatment is not very successful.
-
▪ ▪ Optimal, evidence-based care would cost no more but would increase the health gain by two-thirds.
-
▪ ▪ Even given unlimited funds, three-quarters of the burden of schizophrenia would remain unavertable.
LIMITATIONS
-
▪ ▪ This is a modelling study based on good epidemiology tempered by the many assumptions listed in Table 1.
-
▪ ▪ The potential benefits of non-specific care and treatments yet to be proved are not included, thus the health gain from treatment may be an underestimate.
-
▪ ▪ Optimal, evidence-based treatment presumes treatment concordance by clinician and patient as seen in efficacy studies. This may be optimistic.
Acknowledgement
This paper was supported by the Australian National Health and Medical. Research Council (project no. 113807).












eLetters
No eLetters have been published for this article.