Meta-analytic reviews (Reference Pilling, Bebbington and KuipersPilling et al, 2002; Reference Tarrier and WykesTarrier & Wykes, 2004; Reference Zimmerman, Favrod and TrieuZimmerman et al, 2005) support the efficacy of cognitive–behavioural therapy (CBT) delivered on a one-to-one basis for people with persistent positive psychotic symptoms. Accordingly, recommendations for UK treatment guidelines suggest that CBT should be available for people with schizophrenia (Reference Kendall, Pilling and BarnesKendall et al, 2003). A group format for CBT has been used successfully for a number of disorders (Reference MorrisonMorrison, 2001), pilot studies for group CBT for schizophrenia have reported encouraging results (Reference Gledhill, Lobban and SellwoodGledhill et al, 1998; Reference Wykes, Parr and LandauWykes et al, 1999), and a recent randomised randomised controlled trial of group CBT for people hearing voices reported improvements in hallucinations when therapists were experienced (Reference Wykes, Hayward and ThomasWykes et al, 2005). Seeing that demand for CBT for psychosis is likely to outstrip the availability of trained therapists (Reference Jones, Cormac and Silveira da MotaJones et al, 2005), a group approach might be a useful way of increasing access. Hence, the aim of this study was to evaluate the effectiveness of group CBT for schizophrenia in individuals with persistent positive symptoms.
METHOD
Design
A two-group randomised design was followed. The experimental group received group CBT in addition to standard care, and the control group received standard care alone (treatment as usual). Standard psychiatric care in the UK is based on the care programme approach to case management, and includes maintenance antipsychotic medication, out-patient and community follow-up, and access to community-based rehabilitative activities such as day centres and drop-in centres. Assessments were conducted by independent assessors who were masked to allocation of participants. Although no formal evaluation of the maintenance of the mask was performed, efforts were made to maintain masking, including locating research and therapy staff in separate offices, providing separate locations for assessment and therapy notes, and reminding participants not to disclose their group allocation.
Participants
Ethical agreement for the study was obtained from local research ethics committees. Inclusion criteria were:
-
(a) diagnosis of schizophrenia or schizoaffective disorder verified by case note review, using a checklist for DSM–IV (American Psychiatric Association, 1994) criteria;
-
(b) substance misuse and learning disability not identified as the primary problem;
-
(c) age 18–55 years;
-
(d) persistent and clinically significant positive symptoms, i.e. having either item P3 (hallucinatory behaviour) or item P1 (delusions) from the positive subscale of the Positive and Negative Syndrome Scales (PANSS; Reference Kay, Fiszbein and OpierKay et al, 1987) scored 4 (moderate) or above, with the symptom having been present at this level for at least 50% of the last 2 months;
-
(e) at least 1 month of stabilisation if the patient had experienced a symptom exacerbation in the last 6 months (i.e. at least 1 month since discharge after an acute admission; no change in psychotropic medication prescribed in the last 4 weeks);
-
(f) informed consent from the patient.
Recruitment and randomisation
Potential participants were identified from databases in the five participating National Health Service (NHS) mental health trust sites, and consenting patients were assessed for symptom criteria. Recruitment, randomisation and the running of groups were staggered. Within each site, sufficient participants to form one CBT group and an equal number for the control condition (approximately 12 people) were identified. They were then allocated to the two conditions using a programme operated by an individual independent of the research team, following the minimisation method of stratification (Reference PocockPocock, 1983) for chronicity (3 years or less v. greater than 3 years).
Intervention
The group intervention ran for 6 months, with 18 sessions covering the following themes:
-
• session 1, introduction to the CBT approach to psychosis;
-
• session 2, what is CBT?
-
• session 3, identification of patient problems (delusional beliefs and voices were the main focus);
-
• session 4, formulating problems in terms of thoughts, feelings and behaviours;
-
• session 5, negative thinking patterns and thought monitoring;
-
• sessions 6, 7 and 8, thought challenging;
-
• sessions 9, 10 and 11, behavioural strategies: experiments and action plans;
-
• sessions 12 and 13, stress, arousal and medication;
-
• sessions 14 and 15, staying-well plans;
-
• session 16, emergency staying-well plans;
-
• sessions 17 and 18, follow-up and revision.
Sessions lasted 2 hours including breaks, and followed a detailed plan and timetable contained in the therapy manual (a copy of which can be obtained from the first author). The session plan included setting the day's agenda, introducing the main topic, reviewing homework, applying the topic to individuals’ own experiences, problem formulations in small groups, discussion and comparison of group members’ experiences, setting homework and eliciting feedback on the session.
Treatment quality and adherence
Two therapists conducted each session, and at least one therapist per group had training in CBT meeting the British Association of Behavioural and Cognitive Psychotherapy accreditation standards, plus experience in using CBT with people with psychosis. All therapists were provided with an initial training programme, and supervision sessions occurred monthly. Independent assessment of treatment adherence from audiotaped sessions was not possible because of problems in obtaining consent for taping from all group participants. An alternative measure of treatment adherence (available from the first author) was devised; checklists were completed at each session by both therapists and participants independently, to assess whether key elements of the CBT protocol were adhered to. These elements included agenda-setting, session structure, therapist–patient collaboration, focus on patient cognitions and behaviours, homework-setting and review.
Independently completed checklists from all therapists and participants present were collected on random session dates (20 for participants and 25 for therapists). Interrater reliability was high; there was 92.57% participant agreement and 96.33% therapist agreement. As regards the patient ratings of treatment fidelity, in 164 checklists the percentage of full-adherence scores ranged from 77.4% to 94.5%. For the therapist ratings of treatment adherence 233 checklists were completed. Across all completed checklists, the percentage rated as fully adherent ranged from 86.3% to 94.4%. Hence the checklists indicated that participants and therapists themselves considered they had adhered very closely to the protocol.
Primary outcome measure
This was improvement in positive symptoms as measured by the positive symptom sub-scale of the PANSS. Interrater reliability was assessed on this clinician-rated assessment by computing interclass correlation coefficients for the rating of eight videotaped interviews before starting the trial by the five assessors in this study, and the ratings from gold-standard assessments by four research psychiatrists external to the study. Averaged over the five assessors, the interclass correlation coefficients for the PANSS sub-scales were: positive, 0.84; negative, 0.88; general, 0.71; and total symptoms, 0.91. During the study, random reliability checks were made on ten interviews for each assessor, and average interclass correlation coefficients were: positive, 0.85; negative, 0.84; general, 0.91; and total symptoms, 0.78.
Secondary outcome measures
Secondary interviewer-rated outcome measures included the negative, general and total PANSS scores, and the Global Assessment of Functioning (GAF; American Psychiatric Association, 1987) using the two-scale scores (0–100) of symptoms and disability. Reliability of the interviewers for the latter was assessed using a subsample of 40 participants and two raters. The intraclass correlations were: r=0.96 (symptoms) and r=0.87 (disability).
Secondary self-report outcome measures
These were the Social Functioning Scale (SFS; Reference Birchwood, Smith and CochraneBirchwood et al, 1990); the Hospital Anxiety and Depression Scale (HADS; Reference Zigmond and SnaithZigmond & Snaith, 1983); the Beck Hopelessness Scale (BHS; Beck, 1974); and the Rosenberg Self-Esteem Scale (RSE; Reference RosenbergRosenberg, 1965).
Relapse and readmission
Finally, two methods of assessing the frequency and duration of relapse and readmission to hospital in the 6 months after the treatment period ended (12 months’ follow-up) were measured using definitions from a previous trial (Reference Barrowclough, Tarrier and LewisBarrowclough et al, 1999). These were the number and duration of hospital admissions identified from hospital record systems, and the number and duration of exacerbations of symptoms lasting longer than 2 weeks and requiring a change in patient management (increased observation or medication change made by clinical team as assessed from hospital case notes). Where symptom exacerbation preceded admission to hospital, only one relapse was recorded. Interrater reliability for the number and duration of exacerbations was checked by comparing ratings for ten randomly selected participants. No differences were found between the two independent assessors for these variables.
Strategy for statistical analyses
To minimise the number of missing cases, separate cross-sectional analyses were performed to examine the treatment effects for each outcome measure at 6 months (post-treatment) and 12 months (followup). A linear random effects model adjusted for the outcome measure at baseline, together with age, gender and time since onset. Since treatment administered in a group can create dependencies among observations that violate the independence of observations assumption of statistical tests (Reference Baldwin, Murray and ShadishBaldwin et al, 2005), the model included a random effect to account for the between-group variation, analogous to that used in cluster randomised trials (Reference Roberts and RobertsRoberts & Roberts, 2005). As noted above, within each participating NHS trust patients were randomised in blocks of approximately 12, to permit patients from one locality to form a CBT group and an equal number to experience the control condition. Therefore the analyses also included a random effect for block to prevent between-block variation (due to unknown factors peculiar to that group of patients) inflating the between-treatment arm variation. From the estimates of the variance of random effects, intracluster correlation coefficients were calculated as a measure of the lack of independence resulting from patients being treated in groups. These coefficients would take on a value of zero if there was no intragroup correlation, and one if there was complete concordance in outcome for members of the same group, for all groups.
A longitudinal model was also fitted to the 6- and 12-month data combined. As well as the baseline covariates, this included time point (6 or 12 months), treatment (group CBT or treatment as usual), and a time–treatment interaction as well as random effects for participants and therapy group. In these analyses, a significant time–treatment interaction effect would be interpreted as change in the treatment effect from 6 to 12 months. If there was no interaction, the main effect of treatment would indicate that the treatment effects of group CBT and treatment as usual were similar at 6 and 12 months.
To facilitate comparison between measures and other trials, standardised treatment effects were computed by dividing the treatment effect by the pooled baseline standard deviations for the group CBT and treatment as usual. Finally, relapse outcomes were analysed using a survival model.
RESULTS
Participant recruitment and follow-up
Of 127 people who consented to being screened for eligibility, 113 (89%) fulfilled inclusion criteria and were recruited into the study; ten CBT groups were conducted (Fig. 1).
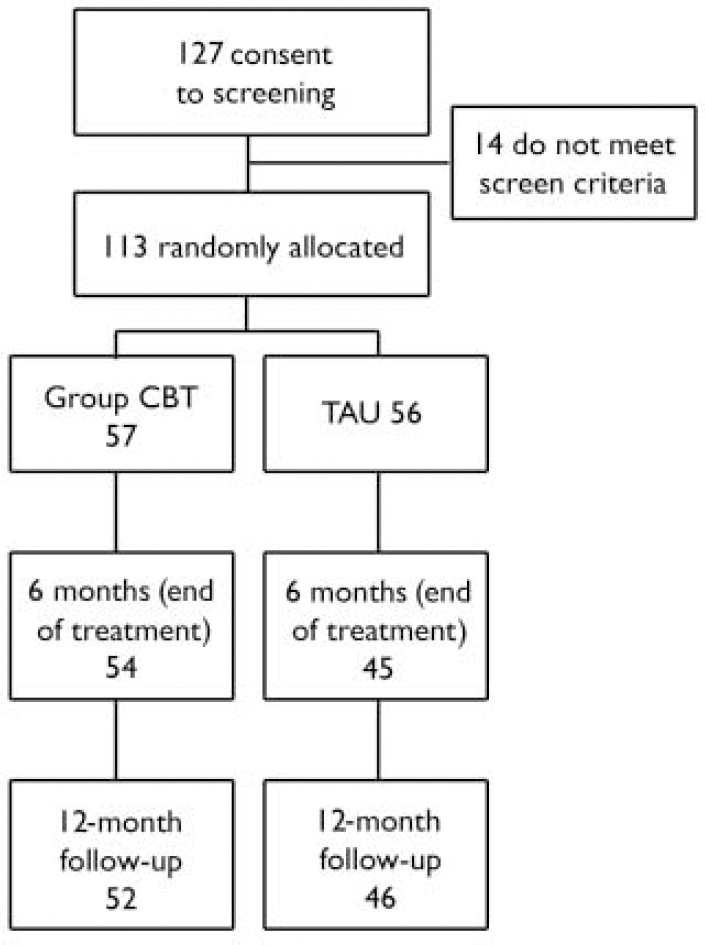
Fig. 1 Flow of participants through the study. CBT, cognitive–behavioural therapy; TAU, treatment as usual.
Of the 113 participants, 57 were allocated to group CBT and 56 to treatment as usual. At the end of treatment (6-month assessment), 99 (88%) participants completed the primary outcome measure (PANSS). These included 54 (95%) of the CBT group and 45 (80%) of the treatment-as-usual group. At follow-up (12 months), 97 (86%) participants completed the PANSS. These included 52 (91%) of the CBT group and 46 (82%) of the treatment-as-usual group.
The mean number of group CBT sessions attended was 10.4 (s.d.=6.5). Using a cut-off for attendance of at least 6 sessions, 41 (72%) of the CBT group could be classed as attenders; 34 (60%) attended 12 or more sessions. All analyses were reported on an intention-to-treat basis, whereby all participants who agreed to assessment were included.
Sample characteristics
Of the total study sample, 82 (72.6%) were men; the mean age of the participants was 38.83 years (s.d.=8.6); the mean illness duration was 13.67 years (s.d.=7.99); 73 participants were single (64.6%), 19 (16.8%) married or cohabiting and 21 (18.6%) separated or divorced; 48 (42.5%) lived alone, 24 (21.2%) lived with a relative or caregiver, 33 (29.2%) lived in a supported hostel or flat and 7 (6.2%) lived in unsupported hostel or other accommodation. The majority of participants (101, 89.1%) were diagnosed with schizophrenia and 12 (10.9%) had a diagnosis of schizoaffective disorder. The mean IQ score estimated from the National Adult Reading Test (NART; Reference Russell, Munro and JonesRussell et al, 2000) scores for the sample was 105.2 (s.d.=11.5). There were no differences between groups on any of the demographic variables assessed.
Outcomes
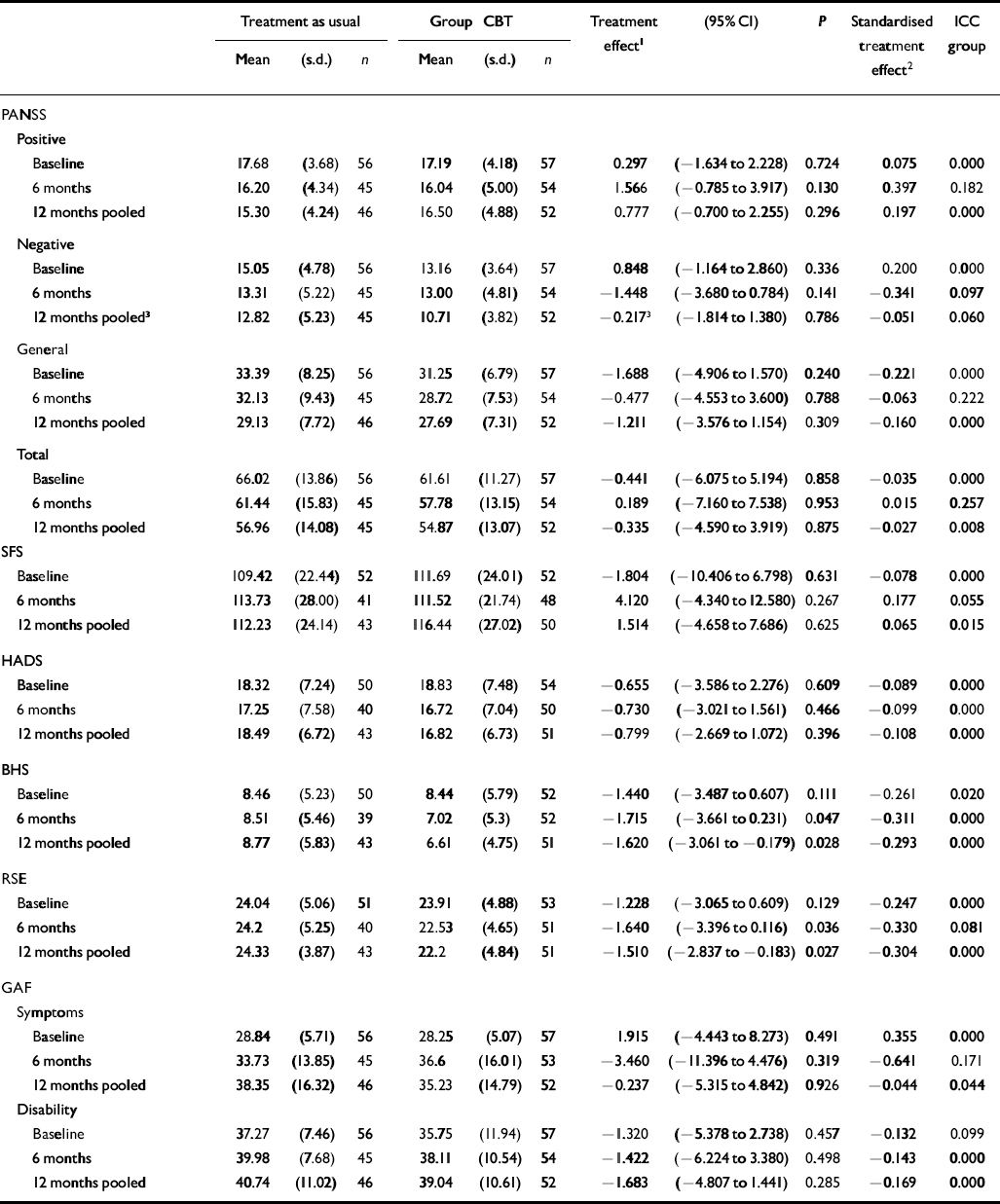
Table 1 gives the summary statistics for the outcome measures, estimates of the treatment effects from the cross-sectional analyses, and the intercluster correlation coefficients for the effects of the groups. For most outcome measures there was little evidence of similarity in outcome due to group membership. There was no evidence of a treatment effect of group CBT as compared with treatment as usual either at completion of treatment or at 1-year follow-up for the PANSS positive sub-scale, nor other PANSS component or total scores. Similarly, group CBT did not appear to affect outcome for SFS total, HADS or the GAF symptom or disability scores. However, there was improvement in the BHS and RSE scores in favour of the group treatment at the 12-month time point.
Table 1 Summary outcome data with estimates of treatment effects
| Treatment as usual | Group CBT | Treatment effect1 | (95% CI) | P | Standardised treatment effect2 | ICC group | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (s.d.) | n | Mean | (s.d.) | n | ||||||
| PANSS | |||||||||||
| Positive | |||||||||||
| Baseline | 17.68 | (3.68) | 56 | 17.19 | (4.18) | 57 | 0.297 | (-1.634 to 2.228) | 0.724 | 0.075 | 0.000 |
| 6 months | 16.20 | (4.34) | 45 | 16.04 | (5.00) | 54 | 1.566 | (-0.785 to 3.917) | 0.130 | 0.397 | 0.182 |
| 12 months pooled | 15.30 | (4.24) | 46 | 16.50 | (4.88) | 52 | 0.777 | (-0.700 to 2.255) | 0.296 | 0.197 | 0.000 |
| Negative | |||||||||||
| Baseline | 15.05 | (4.78) | 56 | 13.16 | (3.64) | 57 | 0.848 | (-1.164 to 2.860) | 0.336 | 0.200 | 0.000 |
| 6 months | 13.31 | (5.22) | 45 | 13.00 | (4.81) | 54 | -1.448 | (-3.680 to 0.784) | 0.141 | -0.341 | 0.097 |
| 12 months pooled3 | 12.82 | (5.23) | 45 | 10.71 | (3.82) | 52 | -0.2173 | (-1.814 to 1.380) | 0.786 | -0.051 | 0.060 |
| General | |||||||||||
| Baseline | 33.39 | (8.25) | 56 | 31.25 | (6.79) | 57 | -1.688 | (-4.906 to 1.570) | 0.240 | -0.221 | 0.000 |
| 6 months | 32.13 | (9.43) | 45 | 28.72 | (7.53) | 54 | -0.477 | (-4.553 to 3.600) | 0.788 | -0.063 | 0.222 |
| 12 months pooled | 29.13 | (7.72) | 46 | 27.69 | (7.31) | 52 | -1.211 | (-3.576 to 1.154) | 0.309 | -0.160 | 0.000 |
| Total | |||||||||||
| Baseline | 66.02 | (13.86) | 56 | 61.61 | (11.27) | 57 | -0.441 | (-6.075 to 5.194) | 0.858 | -0.035 | 0.000 |
| 6 months | 61.44 | (15.83) | 45 | 57.78 | (13.15) | 54 | 0.189 | (-7.160 to 7.538) | 0.953 | 0.015 | 0.257 |
| 12 months pooled | 56.96 | (14.08) | 45 | 54.87 | (13.07) | 52 | -0.335 | (-4.590 to 3.919) | 0.875 | -0.027 | 0.008 |
| SFS | |||||||||||
| Baseline | 109.42 | (22.44) | 52 | 111.69 | (24.01) | 52 | -1.804 | (-10.406 to 6.798) | 0.631 | -0.078 | 0.000 |
| 6 months | 113.73 | (28.00) | 41 | 111.52 | (21.74) | 48 | 4.120 | (-4.340 to 12.580) | 0.267 | 0.177 | 0.055 |
| 12 months pooled | 112.23 | (24.14) | 43 | 116.44 | (27.02) | 50 | 1.514 | (-4.658 to 7.686) | 0.625 | 0.065 | 0.015 |
| HADS | |||||||||||
| Baseline | 18.32 | (7.24) | 50 | 18.83 | (7.48) | 54 | -0.655 | (-3.586 to 2.276) | 0.609 | -0.089 | 0.000 |
| 6 months | 17.25 | (7.58) | 40 | 16.72 | (7.04) | 50 | -0.730 | (-3.021 to 1.561) | 0.466 | -0.099 | 0.000 |
| 12 months pooled | 18.49 | (6.72) | 43 | 16.82 | (6.73) | 51 | -0.799 | (-2.669 to 1.072) | 0.396 | -0.108 | 0.000 |
| BHS | |||||||||||
| Baseline | 8.46 | (5.23) | 50 | 8.44 | (5.79) | 52 | -1.440 | (-3.487 to 0.607) | 0.111 | -0.261 | 0.020 |
| 6 months | 8.51 | (5.46) | 39 | 7.02 | (5.3) | 52 | -1.715 | (-3.661 to 0.231) | 0.047 | -0.311 | 0.000 |
| 12 months pooled | 8.77 | (5.83) | 43 | 6.61 | (4.75) | 51 | -1.620 | (-3.061 to -0.179) | 0.028 | -0.293 | 0.000 |
| RSE | |||||||||||
| Baseline | 24.04 | (5.06) | 51 | 23.91 | (4.88) | 53 | -1.228 | (-3.065 to 0.609) | 0.129 | -0.247 | 0.000 |
| 6 months | 24.2 | (5.25) | 40 | 22.53 | (4.65) | 51 | -1.640 | (-3.396 to 0.116) | 0.036 | -0.330 | 0.081 |
| 12 months pooled | 24.33 | (3.87) | 43 | 22.2 | (4.84) | 51 | -1.510 | (-2.837 to -0.183) | 0.027 | -0.304 | 0.000 |
| GAF | |||||||||||
| Symptoms | |||||||||||
| Baseline | 28.84 | (5.71) | 56 | 28.25 | (5.07) | 57 | 1.915 | (-4.443 to 8.273) | 0.491 | 0.355 | 0.000 |
| 6 months | 33.73 | (13.85) | 45 | 36.6 | (16.01) | 53 | -3.460 | (-11.396 to 4.476) | 0.319 | -0.641 | 0.171 |
| 12 months pooled | 38.35 | (16.32) | 46 | 35.23 | (14.79) | 52 | -0.237 | (-5.315 to 4.842) | 0.926 | -0.044 | 0.044 |
| Disability | |||||||||||
| Baseline | 37.27 | (7.46) | 56 | 35.75 | (11.94) | 57 | -1.320 | (-5.378 to 2.738) | 0.457 | -0.132 | 0.099 |
| 6 months | 39.98 | (7.68) | 45 | 38.11 | (10.54) | 54 | -1.422 | (-6.224 to 3.380) | 0.498 | -0.143 | 0.000 |
| 12 months pooled | 40.74 | (11.02) | 46 | 39.04 | (10.61) | 52 | -1.683 | (-4.807 to 1.441) | 0.285 | -0.169 | 0.000 |
In the longitudinal analyses, there was no evidence of a time–treatment interaction except for the variable PANSS negative symptoms scores where results were of borderline significance (P=0.054). From examination of Table 1, it can be seen that the group CBT treatment effect for PANSS negative symptom scores changed from a very slight detrimental effect at 6 months to a larger beneficial, but still non-significant, effect at 12 months. When models were fitted without an interaction term, there was evidence of a significant effect in favour of the group treatment in the pooled estimate for BHS (P=0.028) and RSE (P=0.027), but not for other measures.
As regards relapse outcomes, data on relapse were gathered for 110 of the original 113 participants in the study–1 patient in the treatment-as-usual group died and notes were missing for two in the CBT group. At the end of the 12-month followup period, 18 members of the CBT group had had at least one relapse (32.7%) compared with 15 (27.3%) in the treatment-as-usual group (χ2=0.82, P=0.365).
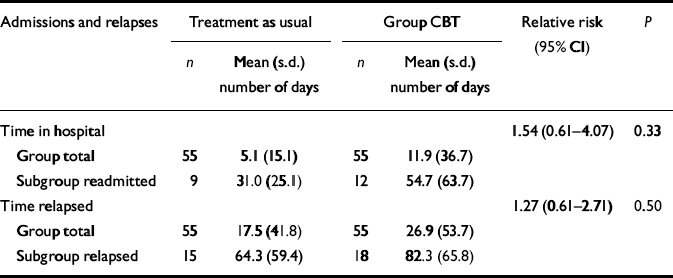
There were no differences between the two groups in terms of number of days in hospital (CBT median=0, range=0–181; treatment-as-usual median=0, range=0–88; z=0.14, P=0.887), number of days in exacerbation (CBT median=0, range=0–188; treatment-as-usual median=0, range=0–212; z=0.34, P=0.737) and the total number of days in relapse (CBT median=0, range=0–188; treatment-as-usual median=0, range=0–212; z=0.20, P=0.844). Time until relapse or admission was analysed using a Cox proportional hazard model. Robust standard errors were used to adjust for any clustering associated with therapy group. Table 2 gives the relative risk for admission and relapse for the group CBT participants as compared with those in treatment as usual. There was no difference between groups, although the relatively low relapse rates meant that this comparison had low power to detect statistical difference.
Table 2 Hospital admissions and relapse
| Admissions and relapses | Treatment as usual | Group CBT | Relative risk (95% CI) | P | ||
|---|---|---|---|---|---|---|
| n | Mean (s.d.) number of days | n | Mean (s.d.) number of days | |||
| Time in hospital | 1.54 (0.61-4.07) | 0.33 | ||||
| Group total | 55 | 5.1 (15.1) | 55 | 11.9 (36.7) | ||
| Subgroup readmitted | 9 | 31.0 (25.1) | 12 | 54.7 (63.7) | ||
| Time relapsed | 1.27 (0.61-2.71) | 0.50 | ||||
| Group total | 55 | 17.5 (41.8) | 55 | 26.9 (53.7) | ||
| Subgroup relapsed | 15 | 64.3 (59.4) | 18 | 82.3 (65.8) | ||
DISCUSSION
The central hypothesis of the study – that group CBT would produce significant positive psychotic symptom improvement compared with treatment as usual – was not supported by the findings. However, although there were no significant differences between the two groups on measures of symptoms or functioning or relapse, members of the CBT group did report a reduction in feelings of hopelessness and low self-esteem. For the latter outcomes, modest effect sizes of approximately 0.3 were found for the follow-up period.
Why did group CBT fail to improve psychotic symptom outcomes? Is the study's failure to match such outcomes for individually treated patients in previous studies due to methodological differences or weaknesses? Or are factors inherent in the group format not conducive to reducing psychotic symptoms?
Methodological issues
Were the therapists inadequately trained? The recent randomised controlled trial of group CBT for individuals who hear voices (Reference Wykes, Hayward and ThomasWykes et al, 2005) concluded that hallucinations were not reduced unless therapy was conducted by expert therapists. In the current trial, it seems unlikely that failure to replicate good outcome could be accounted for in terms of inferior quality of therapy. A number of the therapists had worked on previous CBT trials for psychosis that had had good symptom outcomes; high standards for training and supervision were adhered to; and measures of treatment fidelity indicated that there were no significant deviations from the treatment protocol.
Did the therapy protocol deviate from that of other CBT and psychosis studies? The therapy protocol followed in our trial met all the inclusion criteria for CBT suggested by the Pilling et al (Reference Pilling, Bebbington and Kuipers2002) metaanalytic review. With a total of 18 2-hour sessions over a 6-month period, it also fell within the longer-term treatments which the review suggests may be associated with a better outcome. However, although attendance at the group treatment was quite good, with 60% attending at least two-thirds of the sessions, the total amount of therapy for some participants may have been inadequate.
Did the sample population differ from that of previous trials? Like several key previous trials (e.g. Reference Kuipers, Garety and FowlerKuipers et al, 1997; Reference Tarrier, Yusupoff and KinneyTarrier et al, 1998) we included only out-patients who were persistently treatment resistant, and all our inclusion criteria were in line with those of previous studies. Our sample was slightly older than the mean age for the six trials reported by Pilling et al (Reference Pilling, Bebbington and Kuipers2002) (38.8 years v. 33.9 years) and contained more men (72.6% v. 60.4%) although there are no indications that these differences would have been meaningful in terms of outcomes.
Was the study methodologically rigorous in terms of measuring outcomes? All the assessors were trained to a reliable standard at the start, and their reliability was monitored throughout the study, so there are no indications that assessment of outcome was not methodologically rigorous. Breaks in masking were not assessed but there is no evidence of bias in favour of the CBT groups since only self-report assessments showed superior outcomes for such therapy. Differences in the delivery and take-up of standard care, including medication adherence, were not measured, although the method of randomisation within each hospital site would most likely have reduced the possibility of between-group differences.
Was the sample size adequate? With an initial 113 participants and relatively little attrition, the study was adequately powered to test for differences in terms of improvement in positive symptoms suggested by the version of the Cochrane Library review that was available at the time the study was planned. However, it falls short of the 70 people per group recommended in the current revision (Reference Jones, Cormac and Silveira da MotaJones et al, 2005). Seeing that in this study most of the intraclass correlation coefficients for patients being treated in groups were very small, the sample size was close to that recommended for maintaining 80% power for treatment in such groups (recommended n=128 for 5 members per group, where intraclass correlation coefficient=0.00, Reference Baldwin, Murray and ShadishBaldwin et al, 2005).
Interpretation of outcome for group CBT for psychosis
Previous published studies of group CBT for schizophrenia reporting positive symptom improvements (Reference Gledhill, Lobban and SellwoodGledhill et al, 1998; Reference Wykes, Parr and LandauWykes et al, 1999) have had small sample sizes, did not have control groups or masked assessment and failed to take account of the potential lack of independence in outcomes of group-treated patients that can increase type 1 errors dramatically (Reference Baldwin, Murray and ShadishBaldwin et al, 2005). The results of the study reported here are consistent with the recently published randomised controlled trial of group CBT for people who hear voices (Reference Wykes, Hayward and ThomasWykes et al, 2005). In that study, there was no impact on auditory hallucinations. There were promising results for secondary outcomes, with a borderline significant advantage to the members of the CBT group for self-esteem and a significant improvement in social functioning.
Wykes et al (Reference Wykes, Hayward and Thomas2005) point out that one clear disadvantage of group work for people with complex problems is that it lacks the flexibility to respond to diverse problem presentations, and they suggest that group CBT for psychosis might be more effective if there were homogeneity of symptom experience. However, in their study, even when the group focused on the common experience of hearing voices, hallucinations were not reduced (although there was some indication that participants treated by more expert therapists fared better).
Participants in our study were surveyed as to the advantages and disadvantages of the groups. Overall, feedback from attendees was very positive, and can be summarised in terms of the opportunity to share difficulties in a supportive context. Negative feedback focused on two sets of issues: factors that would lead to a problem in group dynamics, such as participants being dissimilar in terms of age or gender, and factors which might be seen as interfering with new learning, such as disruptions from agitated patients and inconsistent or poor attendance by some members. These factors also presented problems for therapists, and might be addressed in clinical practice where it is possible to select group participants on the basis of homogeneity of symptoms and demographic characteristics. Unfortunately, these issues were not systematically measured in our study, so their impact on outcomes could not be assessed.
A tentative conclusion is that for people with psychosis who have a broad range of persistent positive symptoms, group is less likely than individual CBT to have an impact on hallucinations and delusions, even when delivered by experienced therapists. The study design did not permit us to assess the contribution of group attendance per se to the outcomes. Factors such as getting out of the house for an afternoon each week, and meeting new people in a supportive environment, may have contributed to any patient gains rather than the specific therapeutic input of the CBT. However, the study demonstrated that group cognitive–behavioural work may have important potential benefits, including feeling less negative about oneself and less hopeless for the future. The importance of these changes should not be underestimated, in view of the prominent role that hope and empowerment have been given in recent models of recovery (Reference Resnick, Fontana and LehmanResnick et al, 2005). Future group work with people who have psychosis may be more effective if it specifically targets outcomes such as affect and self-esteem.
Acknowledgements
We are grateful to the therapists, the assessors and the patients participating in the study.
The study was funded by the National Health Service Executive North West Research and Development Funding and from Pennine Care NHS Trust Research & Development monies.








eLetters
No eLetters have been published for this article.