Cognitive impairment can be found in the majority of patients with schizophrenia (Reference Kelly, Sharkey and MorrisonKelly et al, 2000) and is a strong predictor of eventual social and functional outcome (Reference GreenGreen, 1996). The published research examining the long-term course of impairment is inconsistent. Cross-sectional studies have provided evidence both for (Reference Stratta, Arduini and DaneluzzoStratta et al, 2004) and against (Reference Hyde, Nawroz and GoldbergHyde et al, 1994) progressive deterioration but are vulnerable to cohort effects producing differences not due to within-individual changes. More recently, well-designed longitudinal studies have again produced conflicting results with some supporting the notion of progressive decline (Reference Stirling, White and LewisStirling et al, 2003) and others refuting it (Reference Gold, Arndt and NopoulosGold et al, 1999; Reference Hoff, Sakuma and WienekeHoff et al, 1999). Here we report a study examining the course of schizophrenic cognitive impairment over a period of, on average, 33 years.
METHOD
Until 1977 routine neuropsychological testing was performed on all patients admitted for the first time to Crichton Royal Hospital, Dumfries. Since 1981, repeated censuses have identified all known people in Nithsdale, south-west Scotland, with a diagnosis of schizophrenia for a number of published studies. Of the 182 people with ICD–10 schizophrenia (World Health Organization, 1992) identified in the 1998 census, 48 had their first admission to Crichton Royal Hospital before 1978 and thus had undergone routine neuropsychological testing. Patients with a history of other possible causes of cognitive impairment were excluded. Three potential participants were excluded for this reason and another two declined to participate, thus 43 people with schizophrenia took part: 18 men and 25 women, mean age 61.2 years (s.d.=11.3), mean number of years of education 10 (s.d.=2.2) and mean follow-up interval 32.9 years (s.d.=8). For each participant with schizophrenia three potential controls matched for year of testing, gender and age were identified from hospital psychology records. The current address of only 16 potential controls could be identified, two potential controls had developed a psychotic disorder during the intervening period and were thus excluded and two declined to take part in the study, leaving 12 participating individuals (2 men and 10 women). The diagnoses in the control group were personality disorder (n=5), admitted after overdose (n=4), dissociative motor disorder (n=1) and moderate depressive episode (n=2); the group's mean age was 55.8 years (s.d.=7.9), the mean number of years of education was 10.7 (s.d.=1) and the mean follow-up interval was 30.3 years (s.d.=5.4). Baseline neuropsychological testing was performed by members of the clinical psychology department: for many years this was under the direct supervision of the psychologist (John Raven) who designed the tests. At followup, testing was performed by a trained psychiatrist and testing was not time-limited. Raven's Standard Progressive Matrices (RSPM; Reference RavenRaven, 1958a ) test was administered at baseline and follow-up. This test measures non-verbal abstract reasoning and visuospatial problem-solving abilities; it is a valid measure of general and nonverbal intelligence (Reference LezakLezak, 1995). The Mill Hill Vocabulary Scale (MHVS; Reference RavenRaven, 1958b ) was administered at baseline and follow-up; this has been used in studies of people with schizophrenia and provides a valid and reliable measure of verbal intelligence (Reference LezakLezak, 1995). At follow-up the National Adult Reading Test (Reference NelsonNelson, 1982) was administered as an estimate of premorbid ability, and depressive symptoms were rated using the Zung Depression Rating Scale (ZDRS; Reference ZungZung, 1965). Independent t-test analysis was used to compare the groups on potential confounding variables and Fisher's exact test was used to test for differences in gender ratio. Repeated-measure analyses of covariance (ANCOVAs) were performed on cognitive test scores (patients v. controls) from baseline and follow-up assessments with test–retest interval and ZDRS scores as covariates. Post hoc two-tailed paired t-tests were used to assess intra-individual changes in cognitive test scores.
RESULTS
There was no significant difference between the control and schizophrenia groups on general characteristics. At follow-up testing everyone in the schizophrenia group was receiving antipsychotic medication (typical antipsychotics, n=27; atypical antipsychotics, n=16; 36 were living in the community and 7 were long-stay in-patients. None of the control group was receiving psychotropic medication; all were resident in the community. In the schizophrenia group there was a highly significant difference between estimated premorbid and baseline RSPM (P=0.0004, t=3.86, d.f.=42) and MHVS (P<0.0001, t=7.48, d.f.=42) scores. This difference was not found in the control group.
Repeated-measure ANCOVAs of cognitive test scores at baseline and follow-up with test–retest interval and Zung depression scores as covariates showed a highly significant group×time interaction in RSPM performance over time in participants with schizophrenia compared with controls: F(1,59)=7.895, P=0.007. This differential change over time was not observed for the MHVS scores: F(1,59)=1.017, P=0.318. Changes over time expressed in change per year of follow-up are shown in Fig. 1 (full details of all results available on request).
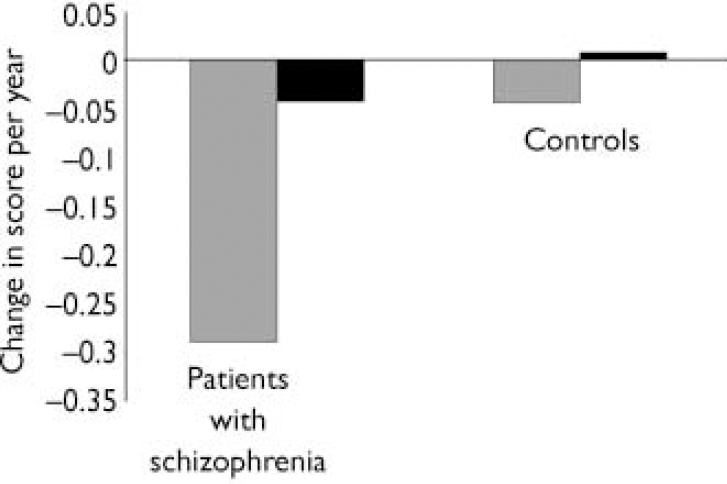
Fig. 1 Change in scores per year of follow-up on Raven's Standard Progressive
Matrices (![]() ;
P=0.007) and on the Mill Hill Vocabulary Scale (▪;
P=0.318).
;
P=0.007) and on the Mill Hill Vocabulary Scale (▪;
P=0.318).
DISCUSSION
This study offers a view of the long-term course of schizophrenic cognitive impairment over an average of 33 years, some 23 years longer than any recent studies. Despite weaknesses inherent in its opportunistic design and difficulties in recruiting controls, we believe it offers interesting results. Participants with schizophrenia showed broad-based cognitive impairment early in their illness, as demonstrated by the significant difference between estimated premorbid scores and actual first-test scores on both the MHVS and RSPM. This difference was not seen in the control groups. Subsequently there was no further significant decline in verbal intelligence as measured by MHVS in the schizophrenia group even over an extended follow-up period, and even though this group arguably might have been particularly severely affected given their continued contact with services and need for antipsychotic medication.
In contrast, there was a significant decline in RSPM scores over time in the schizophrenia group compared with the control group. This could represent a genuine decline, but a number of alternative explanations require consideration. Conditions likely to cause cognitive impairment were specifically excluded in the recruitment process; it is nevertheless possible that some participants had developed a dementia such as Alzheimer's disease. However, the prevalence of Alzheimer's disease in the normal population at this age (mean of the study group was age 61 years) is low and people with schizophrenia do not appear to have a higher incidence of dementia (Reference Purohit, Perl and HaroutunianPurohit et al, 1998). Adverse medication effects might impair cognition; however, there is variability in the reported effects of antipsychotic drugs on cognition (Reference SharmaSharma, 1999). Could the observed decline be due to normal cognitive ageing? Importantly, there was no significant decline in RSPM scores over 30 years in our matched control group. Although to our knowledge there are no longitudinal norms for RSPM over extended periods, a recent re-analysis of sets of RSPM norms collected at different times suggests that little, if any, decline occurs in the normal population between the ages of 20 and 70 years (Reference RavenRaven, 2000). Lastly, testing was not time-limited, thus minimising the effect of slowed psychomotor speed with ageing. These arguments support our belief that the decline in cognitive functions measured by the RSPM in people with schizophrenia (but not observed in our control group) is probably genuine. This is in direct contradistinction to the 5-year longitudinal studies of Gold et al (Reference Gold, Arndt and Nopoulos1999) and Hoff et al (Reference Hoff, Sakuma and Wieneke1999) but is in keeping with a more recent 10-year study showing progressive visuospatial impairment (Reference Stirling, White and LewisStirling et al, 2003), suggesting cognitive decline in schizophrenia occurs over a longer period than 5 years. Our results also suggest that cognitive decline is not global, that is it does not resemble a true dementia, but instead affects specific areas of cognition and may thus reflect ongoing pathological changes in certain areas of the brain or brain systems involved in visuospatial problemsolving and the fluid components of intelligence.






eLetters
No eLetters have been published for this article.