Insomnia is the subjective experience of poor or unrefreshing sleep, usually with some objective evidence of reduced time asleep or delayed sleep onset, although often the subjective experience of suffering may appear more than expected from the degree of sleep shortening. If this occurs in the absence of psychiatric disorder, it is called primary insomnia. Insomnia in other psychiatric disorders is called secondary insomnia and is very common in depression, with up to 90% of patients with sleep disturbance. Sleep abnormalities predict poor response to cognitive–behavioural therapy (CBT) (Reference Thase, Kupfer and BuysseThase et al, 1995) and often continue to disrupt life even when mood has improved, contributing to the risk of relapse. In mania, insomnia often precedes a relapse and may be a useful warning sign of imminent mood swings. The loss of sleep may accelerate the upward mood shift, just as sleep deprivation can elevate mood in people with depression. Insomnia might offer an important signal of impending illness and an opportunity for prophylactic interventions.
DRUG TREATMENT
When measures to improve sleep habits have failed (see Reference Morin and EspieMorin & Espie, 2003), insomnia is usually treated with a variety of drugs – either those that increase brain inhibition through the GABA–A benzodiazepine receptor system or those that decrease excitation through blocking 5-hydroxytryptamine (5-HT) or histamine H1 receptors.
GABA-A receptor drugs
Benzodiazepines and the Z-drugs (zolpidem and zopiclone, and to a lesser extent zaleplon) are the most commonly used hypnotics which act on the benzodiazepine receptor, with the less selective agents clomethiazole and choral hydrate having poor safety profiles. The Z-drugs have a better pharmacokinetic profile than the older benzodiazepines (Reference NuttNutt, 2005), but are equally efficacious (Reference Dundar, Dodd and StroblDundar et al, 2004; http://www.nice.org.uk/TA077) and are the drugs of choice to avoid daytime carry-over effects. Doses are usually halved in older adults (see British National Formulary; http://www.bnf.org) but elderly patients do tend to get up in the night and even short-acting GABA-ergic drugs can compromise balance and cognition early in the night (Reference Allain, tue-Ferrer and PolardAllain et al, 2005). The length of time that hypnotics show efficacy is less clear. In clinical practice many patients are treated with hypnotics for many months or longer. In a placebo-controlled trial of the active (S) enantiomer of zopiclone (eszopiclone) efficacy was maintained over 6 months (Reference Krystal, Walsh and LaskaKrystal et al, 2003) and there are now open-label continuation data for 12 months (Reference Roth, Walsh and KrystalRoth et al, 2005). These long-term controlled data showing continued efficacy of a hypnotic will be reassuring to patients and their treating doctors. Nevertheless, withdrawal reactions with rebound insomnia are still seen in some patients, even with the newer agents, although the timing of these varies with the half-life of the drug. Thus rebound insomnia would be expected on the first drug-free night with drugs with short half-lives or several nights later with drugs with long half-lives.
Antidepressants
Tricyclic and some other classes of antidepressants as well as antipsychotics have long been used for the treatment of insomnia, whereas selective serotonin reuptake inhibitors (SSRIs) generally disrupt sleep early in a course of treatment. This effect of SSRIs on alertness can be offset by sedative antidepressants such as trazodone, probably because they block 5-HT2 receptors which are being overstimulated by an increase in 5-HT (Reference Kaynak, Kaynak and GozukirmiziKaynak et al, 2004). Other 5-HT2 antagonist antidepressants such as nefazodone (Reference Hicks, Argyropoulos and RichHicks et al, 2002) and mirtazapine (Reference Winokur, DeMartinis and McNallyWinokur et al, 2003) have been shown to reduce insomnia in depression, especially early in treatment. There are no controlled studies of the hypnotic efficacy of low-dose amitriptyline but despite this it is fairly common in primary care practice to use 10 or 25 mg amitriptyline to promote sleep. At this dose amitriptyline is probably acting mostly as a histamine H1 receptor antagonist, although a degree of 5-HT2 and cholinergic muscarinic antagonism may also contribute.
Antihistamines
Antihistamines have sedative effects and are sold over the counter as sleeping medications. There is limited evidence that over-the-counter antihistamines work, although recently some benefits have been reported for diphenhydramine in mild insomnia. More profound effects on sleep have been reported for both promethazine and hydroxyzine, although neither is available over the counter and they have quite long half-lives so are likely to cause hangover.
Antipsychotics
For decades sedative antipsychotics, especially thioridazine and chlorpromazine, were used to treat more serious insomnia. More recently, as the cardiac safety of these drugs – especially thioridazine – has been questioned, atypical antipsychotics have been used in this role (most usually olanzapine and quetiapine). These act by blocking 5-HT2 and H1 receptors as well as muscarinic and α1-adrenoceptors, all of which contribute to sedation, but there is no published controlled trial of atypical antipsychotics in insomnia.
Melatonin receptor drugs
Melatonin is a hormone that helps to regulate circadian rhythms. There is a strong public perception of melatonin as a sleep-promoting agent, which leads many people to self-treat with supplies obtained over the internet or from other countries. However, despite its obvious appeal, clinical efficacy data in primary insomnia are very slight (see Reference Buscemi, Vandermeer and HootonBuscemi et al, 2005), although there is some evidence of efficacy in disorders such as jet lag and delayed sleep phase syndrome. Melatonin is also prescribed quite often by child psychiatrists because it seems to have good tolerability, but its efficacy has not been proven in large controlled studies (Reference Buscemi, Vandermeer and HootonBuscemi et al, 2006). One such study is being conducted at present by the National Health Service Technology Assessment Programme. In 2005, a synthetic analogue of melatonin, ramelteon, was granted a licence for the treatment of insomnia in the USA. Ramelteon acts in the same way as the natural hormone to stimulate melatonin receptors and has been shown to promote the onset of sleep in placebo-controlled trials (Reference Erman, Seiden and ZammitErman et al, 2006).
PSYCHOLOGICAL THERAPY FOR INSOMNIA
Psychological therapies for insomnia have been shown to be effective (Reference Morin, Bootzin and BuysseMorin et al, 2006; Reference Espie, MacMahon and KellyEspie et al, 2007). Once a person experiences insomnia, worry about sleep itself often perpetuates the problem. Currently used psychotherapy for insomnia is a combination of behavioural and cognitive strategies which are given to individuals (Reference Jacobs, Pace-Schott and StickgoldJacobs et al, 2004) or to groups (Reference Jansson and LintonJansson & Linton, 2005). The behavioural approaches reinforce natural sleep-initiating and maintaining processes, such as sticking to routines, minimising time in bed spent awake (so limiting rumination) and lowering physical and psychological arousal at bedtime. Cognitive therapy is based on knowledge of the presence of pre-sleep cognitions in patients who find it difficult to get to sleep. The CBT challenges these thoughts to bring about cognitive restructuring.
Cognitive–behavioural therapy for the treatment of insomnia has been evaluated in a number of studies with varying results, probably because of differences in patient groups and the nature, duration and setting of the treatment. Most reports reveal some improvements, which although modest in terms of sleep onset time and total sleeping time, are often greater in areas such as increased quality of life and decreased anxiety about sleep (Reference Green, Hicks and WeeksGreen et al, 2005).
A number of practical issues need to be considered when organising and evaluating these treatments, including selection of participants, access during working hours (many people with insomnia still work) and willingness to engage in the group. Therapists trained in CBT are rarely available as they are usually fully committed to patients with severe mental illness. However, CBT is effective and if available locally should be used for chronic insomnia. It is important to note that a person need not be off medication for the treatment to work.
CONCLUSIONS
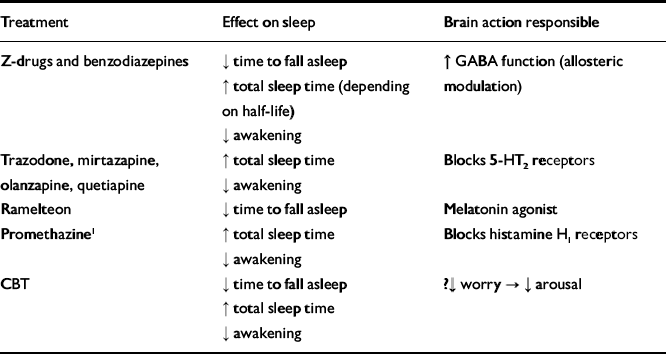
Insomnia is common, especially in people with psychiatric disorder. It is often persistent and disabling and contributes to poorer treatment outcomes and lower quality of life. A summary of treatments for insomnia with their mechanisms of action is given in Table 1.
Table 1 Efficacy and mechanisms in the treatment of insomnia
| Treatment | Effect on sleep | Brain action responsible |
|---|---|---|
| Z-drugs and benzodiazepines | ↓ time to fall asleep | ↑ GABA function (allosteric modulation) |
| ↑ total sleep time (depending on half-life) | ||
| ↓ awakening | ||
| Trazodone, mirtazapine, olanzapine, quetiapine | ↑ total sleep time | Blocks 5-HT2 receptors |
| ↓ awakening | ||
| Ramelteon | ↓ time to fall asleep | Melatonin agonist |
| Promethazine 1 | ↑ total sleep time | Blocks histamine H1 receptors |
| ↓ awakening | ||
| CBT | ↓ time to fall asleep | ?↓ worry → ↓ arousal |
| ↑ total sleep time | ||
| ↓ awakening |
CBT, cognitive-behavioural therapy
1. Only one, single-night, single-dose study available
Current hypnotics that act as agonists of the GABA–A benzodiazepine receptor system, especially the Z-drugs, are safe and effective and new data with eszopiclone shows efficacy for 6 months. However, some people do have trouble in stopping these drugs, owing in part to rebound. Antidepressants with 5-HT2 and H1 receptor-blocking properties are useful for promoting sleep, but tricyclic antidepressants are considerably less safe in overdose so should be used with caution. Antipsychotics have a role in severe insomnia associated with other psychiatric disorders, especially depression and psychosis.
Melatonin has some utility in sleep phase disorders (e.g. jet lag). The new synthetic analogue of melatonin, ramelteon, is effective in sleep-onset insomnia. Psychotherapeutic approaches, especially CBT, can be effective for primary insomnia and can work well in group settings.





eLetters
No eLetters have been published for this article.