There is substantial evidence indicating that a consequence of treatment with some antipsychotic drugs is metabolic dysregulation manifesting particularly as weight gain, potentially resulting in obesity, and impaired glucose tolerance, which may develop into type 2 diabetes. These effects of antipsychotic drugs are likely to contribute to an increased incidence of metabolic syndrome, which identifies a set of obesity-related risk factors for chronic metabolic and cardiovascular disease.
The mechanisms underlying antipsychotic drug-induced metabolic disturbances remain unclear, although genetic predisposition is likely to play an important part in the substantial differences between individuals in the susceptibility to these side-effects. Of the genetic risk factors for antipsychotic-induced metabolic dysregulation, only those associated with weight gain have been investigated to any extent. The strongest evidence lies with the −759C/T polymorphism of the serotonin (5-hydroxytryptamine) 5-HT2C receptor gene for which an association with antipsychotic-induced weight gain has been identified. Reference Reynolds, Zhang and Zhang1–Reference Miller, Ellingrod, Holman, Buckley and Arndt4 This has some a priori validity since antagonism at the 5-HT2C receptor is likely to be a mechanism contributing to this side-effect of antipsychotic drug treatment. Reference Reynolds5
A further gene showing an association with drug-induced weight gain is that for leptin; the functional promoter polymorphism −2548A/G is also associated with long-term development of antipsychotic drug-induced weight gain. Reference Templeman, Reynolds, Arranz and San2 Leptin is an important hormone regulating adipose tissue mass and body weight; it inhibits food intake and stimulates energy expenditure. Thus, although there is no report of a direct genetic association with development of metabolic syndrome in patients treated for schizophrenia, 5-HT2C receptor and leptin gene polymorphisms provide strong candidates.
Method
Participants
Patients with a DSM–IV diagnosis of schizophrenia or schizoaffective disorder currently receiving antipsychotic drug therapy participated in the study. 6 All patients gave written informed consent to the procedures of the study, which was approved by the local research ethics committee. Participants were recruited from two out-patient clinics by asking all those with a diagnosis of schizophrenia or schizoaffective disorder to participate. All those who consented were included. Each participant underwent a detailed interview about personal disease history and family psychiatric disease, diabetes and smoking history. All were tested for random blood glucose level, cholesterol, high- and low-density lipoproteins (HDL, LDL), and triglycerides; blood pressure and waist circumference were measured and body mass index (BMI) calculated from weight and height. Patients with random blood glucose concentrations below 7.8 mmol/l were reassessed; patients with levels consistently above 11.1 mmol/l were classified as having diabetes; those with levels consistently above 7.8 mmol/l underwent a glucose tolerance test, with plasma glucose measured 2 h after 75 g oral glucose: those with levels above 11.1 mmol/l were classified as having diabetes and those with levels above 7.8 mmol/l but below 11.1 mmol/l were classified as having impaired glucose tolerance.
The metabolic syndrome was defined using the International Diabetes Federation criteria (www.metabolic-syndrome-institute.org/news/2006/2006-05-03.2.php) by the presence of abdominal obesity, i.e. waist circumference 94 cm or more in men and 80 cm or more in women, and at least two further risk factors from the following:
-
(a) raised triglycerides (⩾1.7 mmol/l) or specific treatment for this lipid abnormality;
-
(b) reduced HDL cholesterol (<1.03 mmol/l in men and <1.29 mmol/l in women) or specific treatment for this lipid abnormality;
-
(c) raised blood pressure (systolic ⩾130 mmHg or diastolic ⩾85 mmHg) or treatment of previously diagnosed hypertension;
-
(d) raised fasting blood glucose (⩾5.6 mmol/l) or previously diagnosed type 2 diabetes.
Genetic analysis
Genomic DNA was prepared from peripheral blood using standard techniques. All genotyping was carried out strictly blind to the clinical status of the patients. The 5-HT2C receptor polymorphism (–759C/T) and leptin polymorphism (–2548A/G) were determined using a polymerase chain reaction based on the protocol described by Templeman et al. Reference Templeman, Reynolds, Arranz and San2
Statistical analysis
Statistical analysis was performed using SPSS version 11.0 for Windows. Univariate analysis of variance (ANOVA) was used to determine any association between genotype and weight, waist circumference, BMI, blood glucose, triglycerides and cholesterol measures. Chi-squared analysis or, with small numbers where appropriate, Fisher's exact test was used to determine the association between categorical measures including genotype, presence/absence of metabolic syndrome, obesity and smoking.
Results
Case collection
A total of 134 patients (87 men, 47 women), mean age 41.6 years (s.d.=11.8), were recruited to the study. Of these, 121 patients received antipsychotic drug monotherapy. The main oral drug treatments were clozapine (n=21), olanzapine (n=31) and risperidone (n=16); depot medication was received by 27 patients. Two patients were not receiving antipsychotic drug treatment. Assessment of obesity was obtained in 133 participants and glucose metabolism in 131 participants; tobacco smoking was reported in 90 of 134 participants. In the absence of the fasting plasma glucose criterion for the majority of the sample, it proved possible to determine presence or absence of metabolic syndrome in 120 participants.
Metabolic findings
Central obesity was present in 99 of 133 participants (74.4%), defined by increased waist circumference. Stricter criteria (BMI >30 m/kg2) defined 55 of 133 (41%) participants with obesity. Central obesity was significantly more frequent in women (40/47 v. 59/86 in men, P<0.05). Diabetes was present in 6 participants; 2 further individuals had impaired glucose tolerance (6%). These patients were receiving olanzapine (n=3), zotepine (n=2), clozapine (n=1), amisulpride (n=1) or flupenthixol (n=1). Forty-six of 120 patients (38%) had metabolic syndrome.
Gender was significantly related to BMI (P=0.005) and the categorical measures of central obesity (P=0.037) and obesity defined by the BMI >30 kg/m2 criterion (P=0.012), with a greater incidence of obese women, but was not significantly related to metabolic syndrome. Age had no significant effect on measures of obesity but was significantly and positively related to metabolic syndrome (P=0.003). However, the mean age of patients receiving olanzapine (37.6 years) and clozapine (36.2 years) was significantly lower than that of the remaining patients (P<0.001) and the risperidone group (44.2 years; P=0.013); thus age was included as a covariate in further analysis of drug effects.
Tobacco smoking was not significantly related to central obesity or other individual metabolic measures. However, it was significantly related to metabolic syndrome (P=0.047), where smoking was associated with an increased frequency of metabolic syndrome (36/81 v. 10/39 non-smokers).
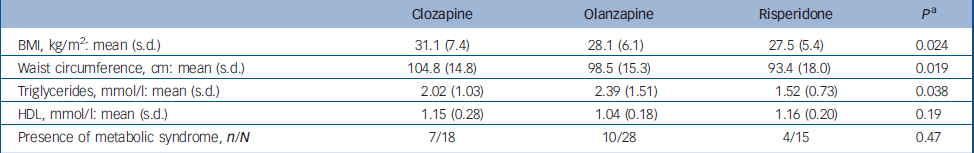
Comparing patients grouped according to the three major drugs prescribed, a significant treatment effect was observed for measures of obesity and for blood triglyceride levels, but not for HDL concentration (Table 1). These reflected generally higher values for olanzapine and clozapine in comparison with risperidone. The effect held true when comparing the risperidone group with participants prescribed either of the other two drugs with regard to waist circumference (101.0 cm v. 93.4 cm; P=0.014), BMI (29.3 kg/m2 v. 27.5 kg/m2; P=0.039) and blood triglycerides (2.24 mmol/l v. 1.52 mmol/l; P=0.022). Again, there was no significant difference in prevalence of metabolic syndrome between the olanzapine/clozapine group and the risperidone group (17/46 v. 4/15).
Table 1 Association of the three major atypical antipsychotic drugs with measures of obesity and metabolic syndrome
| Clozapine | Olanzapine | Risperidone | P a | |
|---|---|---|---|---|
| BMI, kg/m2: mean (s.d.) | 31.1 (7.4) | 28.1 (6.1) | 27.5 (5.4) | 0.024 |
| Waist circumference, cm: mean (s.d.) | 104.8 (14.8) | 98.5 (15.3) | 93.4 (18.0) | 0.019 |
| Triglycerides, mmol/l: mean (s.d.) | 2.02 (1.03) | 2.39 (1.51) | 1.52 (0.73) | 0.038 |
| HDL, mmol/l: mean (s.d.) | 1.15 (0.28) | 1.04 (0.18) | 1.16 (0.20) | 0.19 |
| Presence of metabolic syndrome, n/N | 7/18 | 10/28 | 4/15 | 0.47 |
BMI, body mass index; HDL, high-density lipoprotein
a. Main effect of medication from a univariate analysis of variance with age included as a covariate and, for waist circumference and BMI, gender as a further factor
Genetic findings
5-HT2C receptor gene polymorphism
As the 5-HT2C receptor gene is linked to the X chromosome, male participants were genotyped hemizygotes C or T with 15 of 84 (18%) having the T allele. Among female participants, 15 of 45 (33%) were heterozygotic; none were homozygous for the T allele.
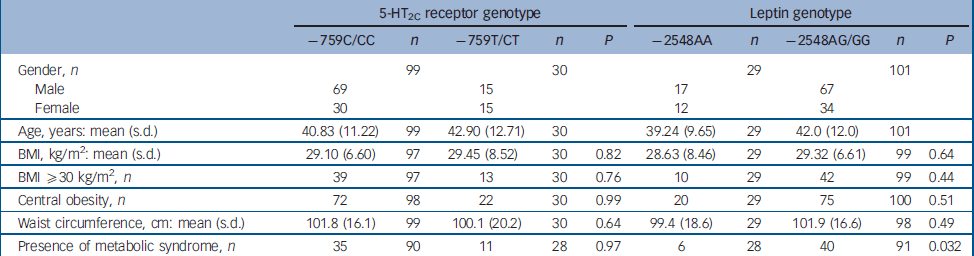
There was no significant association of BMI or waist circumference in the patients with the −759C/T genotype; in addition, gender as a cofactor had no effect on the analysis. Similarly, the presence of obesity and metabolic syndrome showed no significant association (Table 2).
Table 2 Effect of −759C/T 5-HT2C receptor and −2548A/G leptin gene promoter polymorphisms on the measurements of obesity and presence of metabolic syndrome
| 5-HT2C receptor genotype | Leptin genotype | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| –759C/CC | n | –759T/CT | n | P | –2548AA | n | –2548AG/GG | n | P | |||||||||
| Gender, n | 99 | 30 | 29 | 101 | ||||||||||||||
| Male | 69 | 15 | 17 | 67 | ||||||||||||||
| Female | 30 | 15 | 12 | 34 | ||||||||||||||
| Age, years: mean (s.d.) | 40.83 (11.22) | 99 | 42.90 (12.71) | 30 | 39.24 (9.65) | 29 | 42.0 (12.0) | 101 | ||||||||||
| BMI, kg/m2: mean (s.d.) | 29.10 (6.60) | 97 | 29.45 (8.52) | 30 | 0.82 | 28.63 (8.46) | 29 | 29.32 (6.61) | 99 | 0.64 | ||||||||
| BMI ≥30 kg/m2, n | 39 | 97 | 13 | 30 | 0.76 | 10 | 29 | 42 | 99 | 0.44 | ||||||||
| Central obesity, n | 72 | 98 | 22 | 30 | 0.99 | 20 | 29 | 75 | 100 | 0.51 | ||||||||
| Waist circumference, cm: mean (s.d.) | 101.8 (16.1) | 99 | 100.1 (20.2) | 30 | 0.64 | 99.4 (18.6) | 29 | 101.9 (16.6) | 98 | 0.49 | ||||||||
| Presence of metabolic syndrome, n | 35 | 90 | 11 | 28 | 0.97 | 6 | 28 | 40 | 91 | 0.032 | ||||||||
BMI, body mass index
Leptin gene polymorphism
The distribution of leptin gene polymorphism was AA (29/130) 22%, AG (55/130) 42%, GG (46/130) 35%; these results did not deviate significantly from Hardy–Weinberg equilibrium. There was no significant association between the leptin genotype and BMI, waist circumference or presence of obesity (Table 2). The effect of leptin genotype on presence of metabolic syndrome was not significant (AA 6/28, AG 23/49, GG 17/42; P=0.083); however, when participants were grouped on the basis of presence or absence of the G allele, there was a significant association with metabolic syndrome (Table 2). There was no significant association of this division of genotype, or division into heterozygotes and homozygotes, with any of the obesity measures tested above.
Combined 5-HT2C receptor and leptin polymorphism analysis
Combined genotype analysis (5-HT2C receptor CT/T v. CC and leptin AA v. AG/GG) showed a strong overall effect (P=0.007) on BMI in which the leptin genotype had no significant main effect, whereas the effect of 5-HT2C polymorphism reached significance (P=0.048), with a strong gene interaction effect (P=0.001). The same strong interaction was observed in the effect of the polymorphisms on waist circumference (P=0.0003), whereas separate gene effects did not show significance. Correction for age or inclusion of gender had no influence on this result. These effects remained true in the subgroup of patients receiving one of the three main oral drugs, with generally higher levels of significance; inclusion of drug subgroup (clozapine/olanzapine v. risperidone) showed this factor to have a significant effect without adversely influencing the association of BMI with the 5-HT2C polymorphism or the gene interaction effect.
In further investigation of this genetic interaction we observed an effect of leptin polymorphism on the main measures of obesity within the more common −759C/CC 5-HT2C subgroup. Carriers of the G allele exhibited significantly higher BMI and waist circumference than their AA counterparts (Table 3). Gender as a cofactor did not substantially influence these associations. Again, these effects remained true in the subgroup of patients receiving the three main oral drugs, inclusion of drug subgroup (clozapine/olanzapine v. risperidone) generally increasing the significance of genetic associations (data not shown). Association of metabolic syndrome with the leptin polymorphism was also significant within the 5-HT2C C/CC genotype (Table 3), where the leptin AA genotype demonstrated a protective effect (2 of 20 participants having metabolic syndrome).
Table 3 The effect of leptin AG/GG v. AA genotype on the measurements of obesity and presence of metabolic syndrome in the −759CC 5-HT2C patient group

| Leptin genotype | ||||||||
|---|---|---|---|---|---|---|---|---|
| –2548AA | n | –2548AG/GG | n | P | ||||
| BMI, kg/m2: mean (s.d.) | 26.09 (5.53) | 20 | 29.89 (6.66) | 77 | 0.021 | |||
| Waist circumference, cm: mean (s.d.) | 93.78 (16.00) | 20 | 103.86 (15.61) | 76 | 0.012 | |||
| Presence of metabolic syndrome, n | 2 | 20 | 33 | 70 | 0.003 | |||
BMI, body mass index
Discussion
The high prevalence of obesity, often associated with insulin resistance, elevated triglycerides, decreased HDL and/or hypertension (the metabolic syndrome), following long-term antipsychotic treatment is a major problem in clinical psychiatry. The frequency of these metabolic side-effects in patients with schizophrenia is usually 2–4 times higher than in the general population, Reference Heiskanen, Niskanen, Lyytikainen, Saarinen and Hintikka7,Reference De Hert, van Winkel, Van Eyck, Hanssens, Wampers, Scheen and Peuskens8 and varies between individuals and ethnic groups. People with metabolic syndrome are at increased risk of coronary heart disease and type 2 diabetes, contributing to the increased mortality in psychiatric patients. Thus, identifying factors associated with increased risk of developing these adverse effects of pharmacotherapy is potentially very valuable.
These results demonstrate that measures of obesity and other metabolic changes contributing to the metabolic syndrome in a cohort of patients with schizophrenia are influenced by several interacting factors. These include modifiable factors such as antipsychotic drug type and smoking, and genetic factors including the promoter polymorphisms of the 5-HT2C receptor and leptin genes. The absence of a measure of fasting plasma glucose concentration is a limitation of the study, and although a rigorous investigation of participants with elevated random glucose measurements was undertaken to identify impaired glucose tolerance, it is possible that there was some underestimation of the number of people with this disorder. Nevertheless, it proved possible to obtain unequivocal assessment of the frequency of metabolic syndrome from the other metabolic and blood pressure criteria in a large proportion of the sample. Thus a high prevalence of metabolic syndrome was found in our sample. This finding was similar to the recent observation from a study of a large cohort of patients with schizophrenia in Belgium, Reference De Hert, van Winkel, Van Eyck, Hanssens, Wampers, Scheen and Peuskens8 identifying a prevalence of 36% using the International Diabetes Federation criteria and indicating that it is at least twice that expected in the general population. The proportion of patients with diabetes or impaired glucose tolerance was greater than that in the local population (reported as 3.06% on the diabetes register), but the study was not powered to identify significant increases or drug-related differences in this factor; the absence of a control group also limits the conclusions we can come to in this respect.
Investigation of the effect of individual drugs on the metabolic measures undertaken here is limited by the fact that this was a naturalistic and cross-sectional, rather than a longitudinal, study. Full information relating to prior drug treatment, including length of time on current medication and past exposure to other antipsychotics, was not collected for all participants. The effect of such variability in drug exposure would inevitably be to increase the variance in any measures that were sensitive to individual drug type, resulting in a possible underestimate of drug-related differences. Nevertheless, in the three largest groups of patients receiving oral medication, we found a significant increase in measures of obesity and an increase in plasma triglyceride concentrations in patients receiving olanzapine or clozapine compared with those receiving risperidone. This is certainly consistent with the well-established observation of greater weight gain in patients receiving olanzapine or clozapine in comparison with those taking risperidone, Reference Allison, Mentore, Heo, Chandler, Capelleri, Infante and Weiden9 and with the specific effects on triglycerides found in a randomised controlled trial in which, after 8 weeks, significant elevations were found following treatment with olanzapine or clozapine, but not following risperidone or sulpiride. Reference Wu, Zhao, Liu, Zhai, Guo, Guo and Tang10 The values in Table 1 may underestimate the relative differences in drug effects, since measures of body fat and triglycerides increase with age in this group, and the patients receiving risperidone tended to be older than those receiving olanzapine or clozapine. These apparent drug-related differences did not, however, translate into a significant difference between drug treatments in the frequency of metabolic syndrome.
There is a high prevalence of cigarette smoking among patients with schizophrenia. It is well-established that smoking is associated with increased cardiovascular disease and lung cancer, and it is likely that this contributes to increases in coronary heart disease, stroke and respiratory tumours in severe mental illness. Reference Osborn, Levy, Nazareth, Petersen, Islam and King11 Smoking can also have an anorexic effect, and it may be that this effect serves to ameliorate drug-induced weight gain. However, we found no evidence for this; there was no apparent effect of smoking on measures of obesity, whereas conversely smoking was a significant risk factor for the occurrence of metabolic syndrome. This is consistent with the observation that smoking increases risk of the development of type 2 diabetes. Reference Wannamethee, Shaper and Perry12
Several genetic studies associate functional −759C/T 5-HT2C receptor gene polymorphism with antipsychotic-induced weight gain. Yuan et al first showed that polymorphisms, including −759C/T, in the upstream regulatory region of the 5-HT2C receptor gene were associated with increases in prevalence of obesity and type 2 diabetes in the general population. Reference Yuan, Yamada, Ishiyama-Shigemoto, Koyama and Nonaka13 Study of the −759C/T 5-HT2C receptor polymorphism in drug-naïve Chinese patients with schizophrenia showed that those with a T allele exhibited significantly less weight gain after 10 weeks of treatment. Reference Reynolds, Zhang and Zhang1 The finding was replicated in a European first-episode psychosis series following longer-term (up to 9 months) treatment with antipsychotics. Reference Templeman, Reynolds, Arranz and San2 This protective effect of the T allele on antipsychotic-induced weight gain has also been shown in some studies of people with chronic illness receiving antipsychotic drug treatment. Reference Ellingrod, Perry, Ringold, Lund, Bever-Stille, Fleming, Holman and Miller3,Reference Miller, Ellingrod, Holman, Buckley and Arndt4 However, in our study there was no significant association of this polymorphism with measures of obesity or metabolic syndrome. Taken with previous findings, this suggests that the gene may have strong effects on initial weight gain, but a lesser influence on the long-term consequences of that weight gain. In contrast, the leptin gene polymorphism studied here may have greater effects on body mass in the longer term. Templeman et al found an association of this −2548A/G polymorphism with weight gain after 9 months of antipsychotic treatment, in which patients who carried the A allele exhibited lower weight gain, although this was not a significant effect at 3 months. Reference Templeman, Reynolds, Arranz and San2 This promoter region polymorphism has been shown to influence leptin secretion and obesity; Reference Hoffstedt, Eriksson, Mottagui-Tabar and Arner14,Reference Mammes, Betoulle, Aubert, Herbeth, Siest and Fumeron15 presence of the −2548G/G genotype has been found to be associated with extreme obesity in a Taiwanese aboriginal population. Reference Wang, Huang, Chang, Ko, Tsai, Liu, Lee and Ko16
We have found a significant association between the −2548A/G leptin gene promoter polymorphism and the occurrence of metabolic syndrome in schizophrenia. Although there was no significant effect of the 5-HT2C receptor gene polymorphism alone on metabolic measures, including the presence of metabolic syndrome, the leptin and 5-HT2C receptor promoter polymorphisms together show a highly significant association, and strong gene–gene interaction term, with both metabolic syndrome and measures of obesity in patients receiving antipsychotic treatment for schizophrenia.
In our study, patients with the AA leptin genotype exhibited a significantly lower frequency of metabolic syndrome in comparison with the AG/GG genotypes. Thus, these data provide further genetic support for the potential importance of leptin in the development of the metabolic syndrome, Reference Franks, Brage, Luan, Ekelund, Rahman, Farooqi, Halsall, O'Rahilly and Wareham17 which indicates an underlying mechanism relating genotype to phenotype. Combined genotype analysis showed that interaction between the leptin and 5-HT2C receptor polymorphisms was highly significant in their influence on the main measures of obesity. This is consistent with reports demonstrating interactions between the effects of leptin and 5-HT neuronal function. Reference Finn, Cunningham, Rickard, Clifton and Steiner18,Reference Hay-Schmidt, Helboe and Larsen19 In the mediation of central leptin-induced anorexia, 5-HT2C receptors have been implicated, since the 5-HT2C receptor antagonist SB 242084 significantly attenuated the reduction in food intake caused by leptin administration. Reference Von Meyenburg, Langhans and Hrupka20
Templeman et al presented evidence that both the 5-HT2C receptor −759C/T and leptin −2548A/G polymorphisms influence antipsychotic-induced weight gain. Reference Templeman, Reynolds, Arranz and San2 They showed that 5-HT2C and leptin promoter polymorphisms, together with age and BMI, account for 26% of the variance in weight gain at 3 months of antipsychotic treatment. In our study, participants carrying the leptin −2548G allele within the ‘high risk’ 5-HT2C receptor gene −759C/CC group showed significantly higher risk of obesity than those with the AA genotype. Similarly, the presence of metabolic syndrome was highly associated with the presence of the G allele in this group. This reflects the combined effect of two risk factors, the 5-HT2C C/CC genotype and the leptin G allele, in the development of metabolic side-effects. Both of these genetic factors are associated with lower concentrations of circulating leptin independent of the effects of drugs, Reference Templeman, Reynolds, Arranz and San2 and we speculate that the genotype-dependent increased risk of metabolic side-effects may relate to a differential sensitivity to leptin signalling and its disruption by antipsychotic drug treatment. Whatever the underlying mechanisms, our findings highlight the importance of interacting genetic factors in determining both obesity and metabolic syndrome in patients with schizophrenia and thus indicate the possible mechanisms underlying antipsychotic-induced metabolic disturbances and the potential for genetic identification of ‘high risk’ individuals. Genotyping for such risk factors may assist both patient and prescriber in their choice of drug treatment, although diet and exercise, in addition to smoking as highlighted here, remain important modifiable factors influencing the metabolic health of those receiving antipsychotic drug treatment.
Acknowledgement
We thank John Liddle for technical assistance.








eLetters
No eLetters have been published for this article.