Increased rates of white-matter hyperintensities have been a frequent finding in magnetic resonance imaging (MRI) studies evaluating individuals with bipolar disorder compared with healthy controls. Reference Hajek, Carrey and Alda1 Such findings have been reported in individuals with the early stages of bipolar disorder as well as in their unaffected relatives, and this has been taken as suggestive that white-matter hyperintensities could reflect abnormal neurodevelopmental processes in bipolar disorder (see Hajek et al Reference Hajek, Carrey and Alda1 for review). White-matter hyperintensities have also been associated with unipolar depression, particularly in late-onset cases. Reference Soares and Mann2 However, other MRI studies of mood disorders have reported negative results. Reference Sassi, Brambilla, Nicoletti, Mallinger, Frank, Kupfer, Keshavan and Soares3 Moreover, there have been conflicting findings regarding the frequency of white-matter hyperintensities in schizophrenia. Reference Persaud, Russow, Harvey, Lewis, Ron, Murray and du Boulay4 Most studies of white-matter hyperintensities have been conducted with small and heterogeneous samples and this might account for the above inconsistencies. We have evaluated the presence of white-matter hyperintensities in a large sample of people with first-episode psychosis, including individuals with non-affective and affective psychoses, and compared them with people who were psychosis-free and from the same geographical areas. We sought to determine whether white-matter hyperintensities can be linked to affective or psychotic symptoms in general, or whether they would be specifically associated with bipolar disorder.
Method
Participants and assessment schedules
The cases for the present study were selected from a sample of 200 individuals with first-episode psychosis who took part in a population-based case–control study investigating the incidence and risk factors for psychotic disorders in São Paulo, Brazil (see Menezes et al Reference Menezes, Scafzufca, Busatto, Coutinho, McGuire and Murray5 and Schaufelberger et al Reference Schaufelberger, Duran, Lappin, Scazufca, Amaro, Leite, de Castro, Murray, McGuire, Menezes and Busatto6 ). Inclusion criteria for people with first-episode psychosis in the neuroimaging arm of the study were:
-
(a) current age between 18 and 50 years
-
(b) residence for 6 months or more in defined geographic areas of São Paulo
-
(c) diagnosis of a functional psychosis according to the DSM–IV 7 (295–298, psychotic codes), as assessed by the Structured Clinical Interview for DSM–IV (SCID). Reference First, Spitzer, Gibbon and Williams8
Cases with psychotic disorders due to a general medical condition or substance-induced psychosis were excluded.
In order to obtain a population-based psychosis-free sample of controls, 102 next-door neighbours matched for age (within 5 years) and gender were also studied. They were screened to exclude the presence of psychotic symptoms using the Psychosis Screening Questionnaire, Reference Bebbington and Nayani9 and interviewed with the SCID for the assessment of other psychiatric disorders. Exclusion criteria were the same as those for the psychosis group.
Other exclusion criteria for both groups were:
-
(a) history of head injury with loss of consciousness
-
(b) presence of neurological disorders or any organic disorders that could affect the central nervous system
-
(c) moderate or severe mental retardation
-
(d) contraindications for MRI scanning.
From the 200 people with first-episode psychosis included in the epidemiological investigation, Reference Menezes, Scafzufca, Busatto, Coutinho, McGuire and Murray5 46 did not meet the inclusion criteria for the neuroimaging part of the study. From the remaining eligible 154 individuals, 15 could not be contacted after their initial clinical assessment, 24 refused to take part in the MRI session, 2 were excluded due to movement-related imaging artefacts at MRI scanning, and 1 could not be included as he was identified by the epidemiological team when the MRI study and assessment of white-matter hyperintensities had already been concluded. There were no significant differences between the group of individuals with first-episode psychosis recruited from the epidemiological sample (n=112) and the individuals that were lost (n=42) with regard to: current age (t-test=–0.99, d.f.=152, P=0.324), gender (χ2=0.91, d.f.=1, P=0.339), number of years of education (t-test=0.34, d.f.=152, P=0.760), monthly income per capita (Mann–Whitney test, P=0.219), employment status (χ2=0.13, d.f.=1, P=0.715) and marital status (χ2=0.54, d.f.=1, P=0.463).
Seventeen additional people with first-episode psychosis living outside the catchment area of the incidence investigation were also studied, resulting in a total sample of 129 individuals with first-episode psychosis.
Individuals with psychosis and controls were screened for substance use with the Alcohol Use Disorders Identification Test (AUDIT) Reference Saunders, Aasland, Babor, de la Fuente and Grant10 and the South Westminster Questionnaire; Reference Menezes, Johnson, Thornicroft, Marshall, Prosser, Bebbington and Kuipers11 diagnoses of substance abuse or dependence were assessed using the SCID. Reference First, Spitzer, Gibbon and Williams8 Handedness was assessed with Annett's Hand Preference Questionnaire. Reference Annett12 Medical history, including data on cerebrovascular risk factors (presence of arterial hypertension or diabetes) and information about antipsychotic drug treatment were obtained from case notes and interviews with individuals and/or family members.
Current psychotic symptom severity was assessed with the Positive and Negative Syndrome Scale (PANSS) Reference Kay, Fiszbein and Opler13 on the day of MRI scanning. For the people with affective psychoses (bipolar disorder or unipolar major depression), current depressive and manic symptoms were also assessed using, respectively, the 31-item Hamilton Rating Scale for Depression (HRSD) Reference Hamilton14 and the Young Mania Rating Scale (YMRS). Reference Young, Biggs, Ziegler and Meyer15
There were 64 people who fulfilled DSM–IV 7 diagnostic criteria for schizophrenia or schizophreniform disorder, 53 who fulfilled criteria for affective psychosis (25 cases with bipolar disorder and 28 with unipolar major depression) and 12 diagnosed under other psychosis categories, including schizoaffective disorder (n=4), brief psychosis (n=7) and psychotic disorder not otherwise specified (n=1). Due to the small number of individuals in the latter categories, they were included only in the white-matter hyperintensity comparisons between the overall psychosis group and controls, but not in comparisons across diagnostic subgroups. Mood symptom ratings (HRSD and YMRS) were not available for 10 people in the affective psychoses subgroups (4 with bipolar disorder and 6 with unipolar major depression), as these were investigated at the outset of the study when those instruments had not yet been incorporated into our clinical battery.
In the schizophrenia/schizophreniform disorder subgroup, 18 individuals (28%) were not using psychotropic medication at the time of MRI scanning; 42 (45%) were taking antipsychotic drugs (66% typical and 33% atypical), 8 (12%) received antidepressant agents, and 6 (9%) were taking mood stabilisers. There were 6 individuals with bipolar disorders (24%) who were not using psychotropic medication; 12 (48%) were taking antipsychotics (75% typical and 25% atypical), 13 (52%) mood stabilisers and 2 (8%) antidepressant agents. Of the individuals with unipolar major depression 7 (25%) were not taking psychotropic medication; 16 (57%) were using antipsychotic drugs (50% typical and 50% atypical), 14 (50%) antidepressant agents and 4 (14%) mood stabilisers. From the 12 people that received other diagnoses (4 schizoaffective disorder, 7 brief psychosis and 1 psychotic disorder not otherwise specified), 6 were not on psychotropic medication, 6 were receiving antipsychotic medications and 4 mood stabilisers.
The study was approved by local ethics committees and all participants gave informed written consent.
Brain Imaging
Magnetic resonance imaging data were acquired using two identical 1.5 T GE Signa systems (General Electric, Milwaukee, Wisconsin, USA). Exactly the same protocol was used, including acquisition of transaxial T2-weighted fast spin-echo images repetition time (TR)=4000 ms, echo time (TE)=80 ms, field of view (FOV)= 24 cm, slice thickness=3 mm, no gap between the slices, matrix size=256×256) for the assessment of white-matter hyperintensities.
Visual analyses of white-matter hyperintensities were performed by an expert neuroradiologist masked to diagnosis (C.C.C) using the Scheltens scale. Reference Scheltens, Barkh of, Leys, Pruvo, Nauta, Vermersch, Steinling and Valk16 This scale affords prevalence estimates, as well as semi-quantitative ratings based on the number and size of hyperintense lesions, thus providing indirect estimates of severity. Scores for white-matter hyperintensities in periventricular regions (frontal caps, band hyperintensities, and occipital caps) range from zero (absent) to one (hyperintensities up to 5 mm) and two (from 6 to 10 mm); in other brain regions (deep subcortical frontal, parietal, temporal and occipital areas; basal ganglia and infratentorial regions), scores range from zero (absent) to one (up to 3 mm and a maximum of 5 lesions), two (6 or more lesions up to 3 mm), three (up to 5 lesions of 4 to 10 mm), four (6 or more lesions of 4 to 10 mm), five (at least one lesion of more than 10 mm) and six (confluent lesions).
Fifty images were randomly selected and rated again for the location and severity of white-matter hyperintensities by another expert neuroradiologist, in order to provide data for interrater reliability calculations. The intraclass correlation coefficient was employed, and total agreement between raters was found for the following Scheltens scale variables: band hyperintensities, occipital periventricular, deep temporal and occipital white-matter hyperintensities, and all basal ganglia and infratentorial regions but globus pallidus. The other intraclass correlation coefficient measures obtained were: frontal periventricular, 0.91; deep frontal, 0.92; deep parietal, 0.81; and globus pallidus, 0.96.
Statistical analyses
Group comparisons of demographic and clinical data were computed using analysis of variance (ANOVA) for continuous variables or χ2-test for categorical variables.
The following three comparisons of the proportion of people presenting with white-matter hyperintensities (total scores, as well as separate rates in different brain regions) were carried out using χ2-tests: overall psychosis group (n=129) v. controls (n=102); affective psychosis subgroup (n=53, including both those with bipolar disorder and those with unipolar depression with psychotic features) v. non-affective psychosis subgroup (n=64, including schizophrenia/schizophreniform disorder) v. control group (n=102); and schizophrenia/schizophreniform disorder (n=64), psychotic bipolar disorder (n=25) and unipolar psychotic depression (n=28) subgroups v. controls. In addition, as the Scheltens scale provides semi-quantitative ratings, we also conducted comparisons of white-matter hyperintensity scores using Kruskal–Wallis tests, in order to investigate the existence of, respectively, group and subgroup differences in severity.
Finally, Spearman coefficients were used in order to assess the presence of significant correlations between severity ratings and clinical measures, including duration of illness, duration of untreated psychosis (defined as the duration of time from the onset of psychotic symptoms up to the beginning of antipsychotic medication intake), duration of exposure to antipsychotic medication, and severity scores on the positive and negative PANSS scores (for the overall psychosis group), the YMRS (for the bipolar disorder subgroup) and the HRSD (for the unipolar depression subgroup). Due to the large number of correlation indices calculated, levels of significance were set at P<0.05 corrected for multiple comparisons using Bonferroni's method.
Results
Demographic and clinical details
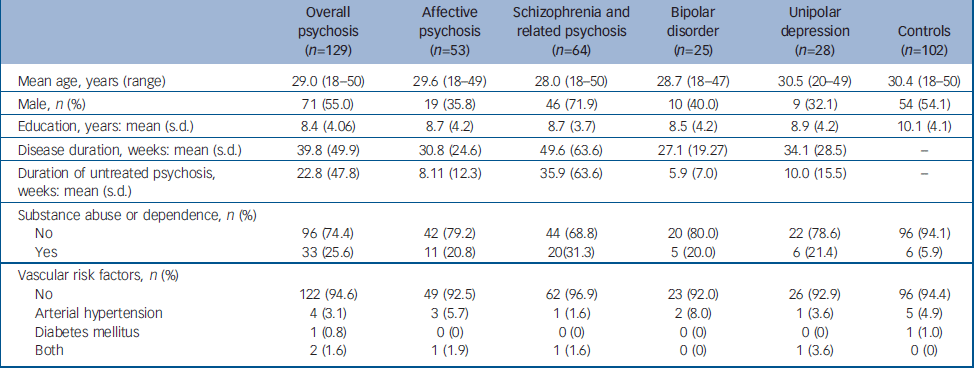
Demographic and clinical data for the psychosis and control groups are summarised in Table 1. Groups (psychosis and controls) and subgroups (affective v. non-affective psychosis v. controls, and schizophrenia/schizophreniform psychosis v. bipolar disorder v. unipolar major depression v. controls) did not differ with regard to mean age, years of education, handedness and presence of vascular risk factors (arterial hypertension and diabetes). There were significantly more males than females in the schizophrenia/schizophreniform psychosis subgroup relative to controls and to all other diagnostic subgroups (χ2 ranging from 5.32 to 15.24, d.f.=1, all P<0.023). The control group also had significantly more males than females relative both to the overall affective psychosis subgroup (χ2=4.56, d.f.=1, P=0.042) and to the unipolar depression subgroup (χ2=4.17, d.f.=1, P=0.055).
Table 1 Demographic and clinical information for participants with psychosis and controls
| Overall psychosis (n=129) | Affective psychosis (n=53) | Schizophrenia and related psychosis (n=64) | Bipolar disorder (n=25) | Unipolar depression (n=28) | Controls (n=102) | |
|---|---|---|---|---|---|---|
| Mean age, years (range) | 29.0 (18-50) | 29.6 (18-49) | 28.0 (18-50) | 28.7 (18-47) | 30.5 (20-49) | 30.4 (18-50) |
| Male, n (%) | 71 (55.0) | 19 (35.8) | 46 (71.9) | 10 (40.0) | 9 (32.1) | 54 (54.1) |
| Education, years: mean (s.d.) | 8.4 (4.06) | 8.7 (4.2) | 8.7 (3.7) | 8.5 (4.2) | 8.9 (4.2) | 10.1 (4.1) |
| Disease duration, weeks: mean (s.d.) | 39.8 (49.9) | 30.8 (24.6) | 49.6 (63.6) | 27.1 (19.27) | 34.1 (28.5) | - |
| Duration of untreated psychosis, weeks: mean (s.d.) | 22.8 (47.8) | 8.11 (12.3) | 35.9 (63.6) | 5.9 (7.0) | 10.0 (15.5) | - |
| Substance abuse or dependence, n (%) | ||||||
| No | 96 (74.4) | 42 (79.2) | 44 (68.8) | 20 (80.0) | 22 (78.6) | 96 (94.1) |
| Yes | 33 (25.6) | 11 (20.8) | 20(31.3) | 5 (20.0) | 6 (21.4) | 6 (5.9) |
| Vascular risk factors, n (%) | ||||||
| No | 122 (94.6) | 49 (92.5) | 62 (96.9) | 23 (92.0) | 26 (92.9) | 96 (94.4) |
| Arterial hypertension | 4 (3.1) | 3 (5.7) | 1 (1.6) | 2 (8.0) | 1 (3.6) | 5 (4.9) |
| Diabetes mellitus | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.0) |
| Both | 2 (1.6) | 1 (1.9) | 1 (1.6) | 0 (0) | 1 (3.6) | 0 (0) |
The psychosis group as a whole showed more comorbid substance abuse or dependence in comparison with the control group (χ2=15.75, d.f.=1, P<0.001). Also, there were significantly more people with comorbid diagnoses of substance abuse or dependence in all three diagnostic subgroups (affective psychosis, schizophrenia/schizophreniform psychosis, bipolar disorder and unipolar depression) relative to controls (χ2 ranging from 5.06 to 19.16, d.f.=1, all P<0.040). No significant differences with regard to comorbid substance abuse or dependence were observed between diagnostic subgroups in paired comparisons (χ2 ranging from 0.01 to 1.64, d.f.=1, all P>0.215).
Frequency and severity
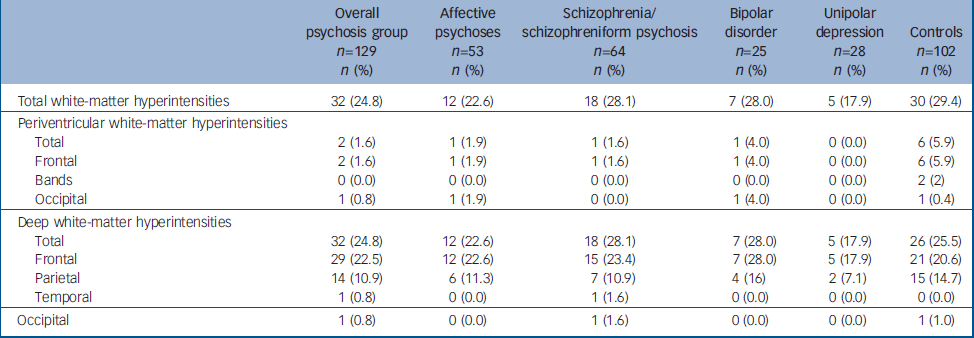
The frequencies of white-matter hyperintensities in each group and subgroup are shown in Table 2. Apart from the rates in deep frontal and parietal regions, scores for all other specific brain regions were very modest or null (Table 2). Therefore, we proceeded with the subsequent statistical analyses only for total white-matter hyperintensity ratings, and for subscores in deep frontal and deep parietal regions (Table 3).
Table 2 Frequencies of white-matter hyperintensities across separate psychosis subgroups and healthy controls
| Overall psychosis group n=129 n (%) | Affective psychoses n=53 n (%) | Schizophrenia/schizophreniform psychosis n=64 n (%) | Bipolar disorder n=25 n (%) | Unipolar depression n=28 n (%) | Controls n=102 n (%) | |
|---|---|---|---|---|---|---|
| Total white-matter hyperintensities | 32 (24.8) | 12 (22.6) | 18 (28.1) | 7 (28.0) | 5 (17.9) | 30 (29.4) |
| Periventricular white-matter hyperintensities | ||||||
| Total | 2 (1.6) | 1 (1.9) | 1 (1.6) | 1 (4.0) | 0 (0.0) | 6 (5.9) |
| Frontal | 2 (1.6) | 1 (1.9) | 1 (1.6) | 1 (4.0) | 0 (0.0) | 6 (5.9) |
| Bands | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2) |
| Occipital | 1 (0.8) | 1 (1.9) | 0 (0.0) | 1 (4.0) | 0 (0.0) | 1 (0.4) |
| Deep white-matter hyperintensities | ||||||
| Total | 32 (24.8) | 12 (22.6) | 18 (28.1) | 7 (28.0) | 5 (17.9) | 26 (25.5) |
| Frontal | 29 (22.5) | 12 (22.6) | 15 (23.4) | 7 (28.0) | 5 (17.9) | 21 (20.6) |
| Parietal | 14 (10.9) | 6 (11.3) | 7 (10.9) | 4 (16) | 2 (7.1) | 15 (14.7) |
| Temporal | 1 (0.8) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Occipital | 1 (0.8) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
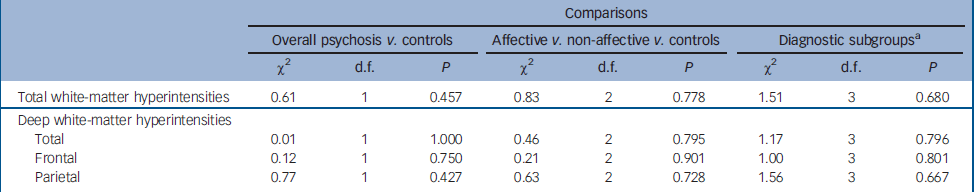
Table 3 Statistical analysis of white-matter hyperintensities across separate pyschosis subgroups and health controls
| Comparisons | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall psychosis v. controls | Affective v. non-affective v. controls | Diagnostic subgroupsa | |||||||
| χ2 | d.f. | P | χ2 | d.f. | P | χ2 | d.f. | P | |
| Total white-matter hyperintensities | 0.61 | 1 | 0.457 | 0.83 | 2 | 0.778 | 1.51 | 3 | 0.680 |
| Deep white-matter hyperintensities | |||||||||
| Total | 0.01 | 1 | 1.000 | 0.46 | 2 | 0.795 | 1.17 | 3 | 0.796 |
| Frontal | 0.12 | 1 | 0.750 | 0.21 | 2 | 0.901 | 1.00 | 3 | 0.801 |
| Parietal | 0.77 | 1 | 0.427 | 0.63 | 2 | 0.728 | 1.56 | 3 | 0.667 |
Using those ratings, no differences in prevalence were observed between: the psychosis group as a whole v. controls; individuals with affective v. non-affective psychoses; or the subgroups with bipolar disorder, unipolar depression and schizophrenia/schizophreniform psychosis v. controls.
Comparisons of scores using Kruskal–Wallis tests revealed no between-group or subgroups severity differences in either of the variables evaluated (online Table DS1). Results remained negative when we repeated such analyses after excluding those people with a positive diagnosis of substance abuse or dependence (20 with schizophrenia/schizophreniform, 5 with bipolar disorder, 6 with psychotic depression and 6 in the control group).
In the entire sample, there were no significant gender differences either in terms of prevalence (χ2 ranging from 0.01 to 1.97, d.f.=1, all P>0.173) or severity (Kruskal–Wallis tests, all P>0.208). There were neither significant differences in prevalence or severity (white-matter hyperintensities: total, total in periventricular regions, total deep, and deep frontal or deep parietal) between people who had a diagnosis of substance abuse or dependence and those who did not (χ2 ranging from 0.11 to 2.90, d.f.=1, all P>0.105; Kruskal–Wallis tests, all P>0.086).
Correlations between scores and clinical variables
No significant correlations were found between white-matter hyperintensity scores and duration of illness, duration of untreated psychosis, duration of exposure to antipsychotic medication or severity of psychotic symptoms – total, positive or negative symptoms (as measured with the PANSS) – in the overall psychosis group (all correlations with associated P≥0.400, corrected for multiple comparisons).
In the bipolar disorder subgroup, there were no significant correlations between the severity of white-matter hyperintensities and the intensity of manic symptoms, as measured by the YMRS (all P=0.068, corrected). Also, no significant correlations were observed between white-matter hyperintensity ratings and HRSD scores in the unipolar depression subgroup (all P=1.00, corrected).
Discussion
The psychosis group and comparisons between affective and non-affective psychoses
The present study is, to our knowledge, the first to systematically evaluate the presence, severity and topography of white-matter hyperintensities in a large group of people with first-episode psychosis compared with healthy controls, using an epidemiological design for the recruitment of the participants. Earlier qualitative investigations cited the possible occurrence of white matter lesions in association with first-episode psychosis (as reviewed by Lawrie et al), Reference Lawrie, Abukmeil, Chiswick, Egan, Santosh and Best17 whereas two recent studies Reference Lubman, Velakoulis, McGorry, Smith, Brewer, Stuart, Desmond, Tress and Pantelis18,Reference Borgwardt, Radue, Götz, Aston, Drewe, Gschwandtner, Haller, Pflüger, Stieglitz, McGuire and Riecher-Rossler19 evaluated the frequency of incidental radiological findings, including white-matter hyperintensities, in individuals with first-episode psychosis through visual non-systematised inspection, with conflicting results.
In the present study, no differences between the overall psychosis group and controls were found in terms of prevalence or severity of these lesions, independently of their brain location. Similarly, no statistically significant differences in the frequencies and severity scores were identified when comparing the affective psychosis (psychotic bipolar disorder and unipolar depression), non-affective psychosis (schizophrenia/schizophreniform disorder) and control subgroups. These results suggest that the presence and extension of white-matter hyperintensities are not associated with vulnerability to psychotic symptoms in adult populations. The absence of significant correlations between white-matter hyperintensity scores and clinical variables related to psychotic features – duration of illness, duration of untreated psychosis and severity of psychotic symptomatology – corroborates this conclusion. It is important, however, to raise the possibility that white-matter hyperintensities could be a feature related to illness chronicity, and this might explain why there were no group differences in our investigation of participants in the early course of their illness. Future longitudinal MRI studies would be needed to elucidate this issue.
Unipolar depression, schizophrenia/schizophreniform psychosis and bipolar disorder
There were no significantly increased rates or severity indices of white-matter hyperintensities in any of the three separate psychosis subgroups relative to the control group.
Our negative findings in the unipolar depression subgroup are in accordance with the most recent MRI studies carried out with samples of non-elderly adults with major depression. Reference Sassi, Brambilla, Nicoletti, Mallinger, Frank, Kupfer, Keshavan and Soares3,Reference Iosifescu, Renshaw, Lyoo, Lee, Perlis, Papakostas, Nierenberg and Fava20 Also, most studies that evaluated the presence of white-matter hyperintensities in schizophrenia have reported negative results, Reference Brown, Lewine, Hudgins and Risch21,Reference Krabbendam, Honig, Wiersma, Vuurman, Hofman, Derix, Nolen and Jolles22 although the literature is conflicting. Reference Persaud, Russow, Harvey, Lewis, Ron, Murray and du Boulay4
Finally, with regard to the bipolar disorder subgroup, the low prevalence of white-matter hyperintensities, indistinguishable from that of the other subgroups, suggests that the magnitude of any supposed white-matter hyperintensity increments in association with bipolar disorder is not sufficiently large to be detected with a sample of the size recruited for the present study, and with the use of a control group selected from the same geographical region. Findings of increased rates in bipolar disorder have been previously reported: two meta-analyses have found that the risk of individuals with bipolar disorder presenting with white-matter hyperintensities is over three times higher than that for healthy controls. Reference Altshuler, Curran, Hauser, Mintz, Denicoff and Post23,Reference Videbech24 In spite of that, several studies have reported negative findings. Reference Sassi, Brambilla, Nicoletti, Mallinger, Frank, Kupfer, Keshavan and Soares3,Reference Brown, Lewine, Hudgins and Risch21,Reference Krabbendam, Honig, Wiersma, Vuurman, Hofman, Derix, Nolen and Jolles22,Reference Chang, Barnea-Goraly, Karchemskiy, Simeonova, Barnes, Ketter and Reiss25 Strakowski et al, Reference Strakowski, Woods, Tohen, Wilson, Douglass and Stoll26 in the only study to date that evaluated frequency in first-episode mania, failed to find a significant difference in comparison with controls, despite reporting a 1.7 higher (non significant) rate of subcortical white-matter hyperintensities in the bipolar disorder group. Moreover, there is considerable heterogeneity in previous reports regarding the prevalence rates in both those with bipolar disorder and control samples, with frequencies of total hyperintensities ranging, respectively, from approximately 5% to 66% and 0% to 58% among studies that evaluated young or adult individuals. Reference Sassi, Brambilla, Nicoletti, Mallinger, Frank, Kupfer, Keshavan and Soares3,Reference Krabbendam, Honig, Wiersma, Vuurman, Hofman, Derix, Nolen and Jolles22,Reference Videbech24,Reference Chang, Barnea-Goraly, Karchemskiy, Simeonova, Barnes, Ketter and Reiss25,Reference Lyoo, Lee, Jung, Noam and Renshaw27,Reference Ahn, Lyoo, Lee, Song, Oh, Hwang, Kwon, Kim, Kim and Renshaw28
The main location where these lesions are found is also controversial, with some studies reporting increased rates of white-matter hyperintensities in periventricular regions in bipolar disorder samples, Reference Altshuler, Curran, Hauser, Mintz, Denicoff and Post23 while others highlight findings of increased deep white-matter hyperintensity ratings associated with bipolar disorder. Reference Videbech24,Reference Lyoo, Lee, Jung, Noam and Renshaw27–Reference McDonald, Tupler, Marsteller, Figiel, DiSouza, Nemeroff and Krishnan29 The only quantitative analysis of white matter lesions in people with psychiatric disorders published to date reported increased volumes of white-matter hyperintensity in anterior brain regions of individuals with bipolar disorder compared with either those with unipolar depression or healthy controls Reference Dupont, Jernigan, Heindel, Butters, Shafer, Wilson, Hesselink and Gillin30 . Although several papers reported frontal Reference Lyoo, Lee, Jung, Noam and Renshaw27,Reference Dupont, Butters, Schafer, Wilson, Hesselink and Gillin31 and frontoparietal Reference Figiel, Krishnan, Rao, Doraiswamy, Ellinwood, Nemeroff, Evans and Boyko32,Reference Aylward, Roberts-Twillie, Barta, Kumar, Harris, Geer, Peyser and Pearlson33 location of white-matter lesions in association with bipolar disorder, only one recent study Reference de Asis, Greenwald, Alexopoulos, Kiosses, Ashtari, Heo and Young34 directly compared frontal deep white-matter hyperintensities between participants with bipolar disorder and controls, in an elderly population, and found increased left frontal scores in the bipolar disorder group. Few authors have systematically assessed vascular risk factors and history of substance abuse or dependence in the participants enrolled, and most studies have been conducted with small and heterogeneous samples, poorly controlled for clinical disease course, age, gender and comorbidities. All these factors, together with methodological differences among studies, are likely to have contributed to the lack of consistency in the findings.
Recent MRI studies using diffusion tensor imaging in people with bipolar disorder, Reference Haznedar, Roversi, Pallanti, Baldini-Rossi, Schnur, Licalzi, Tang, Hollander and Buchsbaum35,Reference Beyer, Taylor, MacFall, Kuchibhatla, Payne, Provenzale, Cassidy and Krishnan36 although as yet scarce and involving modest samples, have revealed structural white matter abnormalities – located in the frontal lobe and adjacently to the striatum and thalamus. Such findings suggest that diffusion tensor imaging techniques may be more sensitive to detect white matter abnormalities in association with bipolar disorder. In support of this possibility, a study combining diffusion tensor imaging and white-matter hyperintensity measurements in healthy elderly individuals detected structural damage evidenced by increased diffusion coefficients (as measured by diffusion tensor imaging) in areas of normally appearing white matter surrounding foci of white-matter hyperintensities. Reference Firbank, Minett and O'Brien37 Although the latter evidence has been found in an elderly population, in which different mechanisms may be involved in the emergence of white-matter hyperintensities, it is possible that they might simply represent the ‘tip of the iceberg’ in terms of structural white matter lesions. Thus, the presence and severity of white-matter hyperintensities associated with bipolar disorder might be understood as an extreme consequence of underlying microstructural processes that affect brain connectivity and which may be more specifically investigated using diffusion tensor imaging methods.
It is possible that some significant findings of our study could have been obscured by factors unrelated to psychiatric diagnoses. For instance, rates of white-matter hyperintensities in the general population are known to increase in direct proportion to the presence of cardiovascular risk factors, particularly in elderly people. Reference Zanetti, Cordeiro and Busatto38 However, we studied a young adult sample with a low prevalence of cerebrovascular risk factors (Table 1). Besides that, no differences in the rates of arterial hypertension and diabetes were observed between people with psychosis and those without, or across the separate diagnostic subgroups. Therefore, we believe that our results are not attributable to group differences in risk factors for cerebrovascular disease. One might also question whether the use of psychotropic medication could influence the presence of white matter lesions. However, there is no evidence that the use of lithium, tricyclic antidepressants or antiepileptic drugs would be linked to increased rates of white-matter hyperintensities. Reference Sassi, Brambilla, Nicoletti, Mallinger, Frank, Kupfer, Keshavan and Soares3,Reference Persaud, Russow, Harvey, Lewis, Ron, Murray and du Boulay4,Reference Altshuler, Curran, Hauser, Mintz, Denicoff and Post23,Reference Videbech24 Data regarding the possible influence of antipsychotic drugs is scarce, but we studied individuals whose psychotic disorders were at an early stage and, therefore, with a short history of exposure to antipsychotic medication. Thus, the probability of any biases due to exposure to such drugs in our study is low.
Limitations
A number of methodological limitations should be highlighted. First, all our participants with bipolar disorder and major depressive disorder presented with psychotic symptoms, and one could argue that these individuals do not adequately represent the whole population suffering from affective disorders. Second, as we employed a semi-quantitative visual analysis method, we cannot discount the possibility that subtler white matter abnormalities may not have been detected. Replication of our results in studies using more quantitative methods for white-matter hyperintensity assessment is, therefore, warranted. Finally, although we studied a sample of individuals with first-onset psychosis of greater size than previous studies, the size of each diagnostic subgroup may have been insufficiently large to avoid the risk of type II errors. Therefore, the findings reported herein should be extended using rating methods of greater sensitivity in larger subgroups.
This study was funded by the Wellcome Trust, UK. P.R.M. and M.S. are partly funded by the CNPq-Brazil.
Acknowledgements
We thank the Brazilian First-Contact Psychosis clinical team for the recruitment of the participants in the community. The authors also thank Leticia Coutinho for her invaluable support in the statistical analyses, Claudia C. Leite and Edson Amaro Jr. for their help in the collection of MRI data, and Zélia M. S. Campos for her contribution in rating the MRI scans for reliability calculations.








eLetters
No eLetters have been published for this article.