Twin and family studies have estimated the heritability of liability to major depression at 30–50% when measured in community samples from high-income countries Reference Sullivan, Neale and Kendler1–Reference Middeldorp, Birley, Cath, Gillespie, Willemsen and Statham4 with a somewhat higher estimate in a hospital-based study. Reference McGuffin, Katz, Watkins and Rutherford5 A range of environmental risk factors may contribute to the remaining variance in liability, including adverse life events, poverty, childhood adversity and physical disease. Examining populations where we might expect the nature, magnitude and frequency of environmental risk factors to vary compared with Western countries may shed light on the identity of true environmental causal factors. However, twin studies outside of North America, Europe and Australia are scarce, despite depression being a pressing public health problem in low- and middle-income countries. Reference Prince, Patel, Saxena, Maj, Maselko and Phillips6 If there are large variations in environmental exposures within a population (e.g. access to basic material resources) we might expect depression to have a lower heritability because a greater proportion of variance is explained by these environmental factors. Alternatively, if a very high proportion of the population is exposed to relevant environmental risks, heritability may be higher (see Rutter et al for a review). Reference Rutter, Moffitt and Caspi7 It is also important to consider that some apparently ‘environmental’ risk factors such as stressful life events show gene–environment correlations, with genetic influences on variation in exposure to such risk factors. Reference Kendler and Karkowski-Shuman8,Reference Thapar, Harold and McGuffin9 In this paper we describe the heritability of depression, and gender differences in heritability, in a population-based twin study in Colombo, Sri Lanka (the Colombo Twin And Singleton Study, CoTASS).
Method
The study received research ethics approval from the Institute of Psychiatry, King's College London, the Ethical Review Committee, University of Sri Jayewardanepura, and the World Health Organization's Research Ethics Committee.
Study design and participants
This was a population-based twin and non-twin sample, although the current study focuses on the twins only. Full details of the design and implementation of the study are described elsewhere. Reference Siribaddana, Ball, Hewage, Glozier, Kovas and Dayaratne10 The study took place in the Colombo District of Sri Lanka, an area with a population of 2.2 million which includes the island's capital and surrounding areas (45% of the district is classed as rural). 11 The survey capitalised on the annual update of the electoral register, which consists of a census of all households conducted by local civil servants. We added a question asking whether the householder knew of any twins, and identified 19 302 individuals who were twins by this method. From these, 4387 participants were randomly selected and were eligible to take part in the project on common mental disorders, of whom 4024 (91.7%) actually participated, including 1954 complete pairs of twins. Individuals were excluded if they were under 15 years of age, failed a Mini-Mental State Examination or when interviews were conducted via a proxy.
The mean age of the participants was 34 years, 46% were male, 53% were married and 90% were of Sinhala ethnicity; the employment rate was 73% for men and 35% for women; the mean number of people in the participants' households was 4.9. 12 The median household income for this region of the country in 2006/7 was 24 711 Sri Lanka rupees (LKR) per month (approximately 233 US dollars), compared with 16 735 LKR for the whole of Sri Lanka. 12 Sri Lanka has more equitable health and social care than South-East Asian neighbours, Reference Jayasekara and Schultz13 and greater gender equality at least as shown in primary and secondary education, 14 and its population experiences considerable variation in poverty, rather than there being a very large economic underclass. Despite the relatively high standards of education, approximately a third had received 10 or fewer years of education (note that the adults in the study may have been of school age several decades ago), whereas almost a third received 13 or more years. Reference Ball, Siribaddana, Kovas, Glozier, McGuffin and Sumathipala15 Life expectancy at birth in Sri Lanka is 69 for men and 76 for women (and ‘healthy life expectancy’ is 59 for men and 64 for women). 16
Interviews took place between 2006 and 2007, when Sri Lanka had been experiencing violent civil war for over 20 years. There have been uprisings and bombing attacks in Colombo, and at times a strong military presence. However, this region has not been as heavily involved as have areas in the north and east of the island. Nonetheless, a small minority (2.6%) of the participants reported directly participating in the conflict as combatants. Similarly, although many people in Colombo have been affected by the tsunami of 2004, direct involvement was not on the same scale as in the south of the island.
Measures
Research workers visited the twins' homes to interview them separately. Interviews were administered including the World Health Organization's Composite International Diagnostic Interview. 17 This gives DSM/ICD diagnoses of mental disorders. For present analyses we used lifetime DSM–IV definitions, 18 excluding opt-outs because of bereavement or mixed states. We also excluded functional impairment criteria (seeking medical help, or describing the symptoms as interfering with life's activities). This was because of the relatively low likelihood that individuals who met symptom criteria also met functional impairment criteria. Reference Ball, Siribaddana, Kovas, Glozier, McGuffin and Sumathipala15 The diagnosis excluding the impairment requirement resulted in a phenotype that was of comparable severity to full DSM depression in Western countries; and within the Sri Lankan sample, the definition excluding an impairment requirement produced an affected group that had very similar symptom characteristics to the affected group according to the full DSM definition. The low reporting of functional impairment may have been as a result of stigma, culturally or economically determined differences in response to depressive symptoms, or the very low proportion of psychiatrists per head of population. Pragmatically, relaxing impairment criteria allowed sufficient power for twin model fitting and there are good precedents for this in recent studies elsewhere (e.g. Kendler et al). Reference Kendler, Gardner, Neale and Prescott2,Reference Kendler, Gatz, Gardner and Pedersen3 The group meeting symptom criteria regardless of impairment is henceforth referred to as the depression group.
In order to assess aspects of depression that may not manifest as full diagnoses, we also examined a broader group who had had at least one ‘core’ DSM symptom of depression most of the day for nearly every day for a minimum of 2 weeks (sad mood, or loss of interest, as a probable psychiatric symptom rather than because of physical illness or drugs). 18 This second definition identifies people who are susceptible to depression, and is referred to as the D-probe group. Both the depression and the D-probe definitions refer to lifetime-ever experiences. The sociodemographic risks were comparable between the D-probe and depression definitions, which were also similar to those for a definition that included functional impairment. Reference Ball, Siribaddana, Kovas, Glozier, McGuffin and Sumathipala15
Zygosity was assessed using a validated questionnaire Reference Ooki, Yamuda, Asaka and Hayakawa19,Reference Sumathipala, De Silva, Siribaddana, Abeysingha and Fernando20 administered to both twins.
Analysis
A database was constructed in SPSS version 14 for Windows. The descriptive statistics were performed in Stata version 9.2 for Windows.
Twin concordance and genetic model fitting
Classic twin studies examine the similarity of monozygotic (MZ) pairs of twins, and compare this to the similarity of dizygotic (DZ) pairs of twins, in order to tease apart the contributions of three factors to individual differences in a phenotype such as depression. These factors are additive genetics (A), shared environments (C) and non-shared environments (E).
The probandwise concordance rate (PWCR) provides a simple description of similarity within pairs of twins for a binary trait such as presence/absence of depression. The PWCR is calculated as the number of individuals in concordantly affected pairs divided by the total number of affected individuals. Thus it is the probability that the co-twin of a proband will also have the disorder, Reference Rijsdijk and Sham21 and describes cross-twin similarity in a way that can be more intuitive than genetic model fitting. The PWCRs were calculated for each gender×zygosity group, assuming complete ascertainment of cases.
We used genetic model fitting in Mx for Windows (www.vcu.edu/mx/index.html) to estimate the relative contributions (and confidence intervals) of A, C and E. Greater similarity (i.e. higher correlations) within MZ than DZ pairs indicates A, since it is assumed that the greater proportion of shared genes is the only explanation for such greater similarity; C includes any environmental exposure that makes twins within a pair (both MZ and DZ) similar to one another (e.g. family-wide poverty); E reflects any environmental factors that make one twin different from the co-twin (e.g. an accident that affects only one twin within a pair), and so it is calculated as the amount of dissimilarity within MZ pairs. Tetrachoric correlations were used because the data are binary. This method assumes that liability to depression is normally distributed throughout the population, with affected individuals having exceeded a certain threshold of liability.
Age and gender are biologically determined factors that cannot be outcomes of depression, but which may index subgroups of people for whom the nature of the disorder and the contributions of aetiological influences (i.e. A, C and E) to the disorder differ. In twin models, gender is commonly accounted for by analysing the male and female groups separately, and testing whether the model parameters can be equated across the gender groups. When analysing the D-probe phenotype, we used a five-group model with groups for MZ male, MZ female, DZ male, DZ female and DZ opposite-gender pairs. However, for depression, the number of concordantly affected male pairs was low, so we used a two-group model (MZ female and DZ female pairs only). The effect of age was accounted for through regression coefficients on the liability thresholds, as has been described previously; Reference Reynolds, Fiske, Fratiglioni, Pedersen and Gatz22 in the current study, these allowed for linear and quadratic effects of age, separately for each gender.
We tested a series of nested models to find the most parsimonious solution. First, we tested for qualitative gender differences, (i.e. whether the identity of the causal genes and environments were the same in men and women), by comparing correlations within same-gender and opposite-gender DZ pairs (because the only factor that is differentially shared is gender). We examined the difference in model fit when: both the genetic correlation and shared environmental correlation for DZ opposite-gender twins are freely estimated; and either the genetic correlation or the shared environmental correlation for DZ opposite-gender twins are fixed to be the same as for DZ same-gender twins (0.5 for the genetic correlation, or 1.0 for the shared environmental correlation). Second, we tested whether the magnitude of A, C and E parameters could be equated across gender groups (quantitative gender differences). Finally, we tested whether any of the ACE parameters could be dropped (if they make no statistically significant contribution to the model). As only females were examined for depression, we used the first and second steps for the D-probe definition only.
Results
Descriptive statistics
The demographic characteristics of the sample are described in detail elsewhere, Reference Siribaddana, Ball, Hewage, Glozier, Kovas and Dayaratne10 but briefly, the average age was 34.0 years, and included 46.2% men, 42.5% MZ pairs, 29.5% DZ same-gender pairs and 27.3% DZ opposite-gender pairs. Depression was present in 8.2% of men and 13.6% of women; D-probe was present in 13.9% of men and 17.3% of women (Table 1). The prevalences varied by age, with the highest prevalences occurring in middle age (e.g. the prevalence of D-probe in ascending age quartiles was 10.0%, 16.5%, 18.9% and 17.3%, F = 10.12, P<0.01).
Table 1 Prevalence of lifetime depression and D-probe
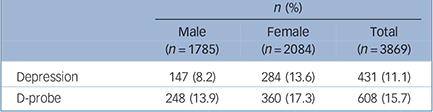
| n (%) | |||
|---|---|---|---|
| Male (n = 1785) | Female (n = 2084) | Total (n = 3869) | |
| Depression | 147 (8.2) | 284 (13.6) | 431 (11.1) |
| D-probe | 248 (13.9) | 360 (17.3) | 608 (15.7) |
Aetiology
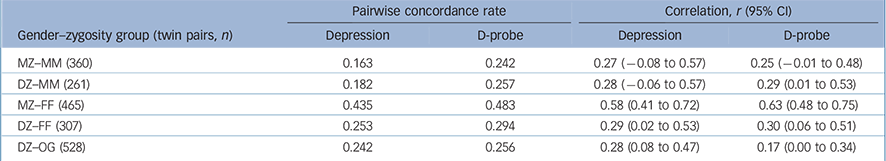
Among females, the PWCRs for both depression and D-probe were higher in the MZ than the DZ same-gender groups (Table 2), indicating genetic influences. However, in males the MZ concordance was no higher than that of the DZ same-gender pairs for either depression phenotype. This suggests that shared environmental (C) rather than genetic influences (A) are driving familial resemblance. Non-shared environmental factors are indicated in both genders since the MZ probandwise concordance is short of 1.0. These findings are also reflected in the polychoric correlations (Table 2), which are the basis of the model fitting.
Table 2 Probandwise concordance rates (prior to age adjustment) and tetrachoric correlations (estimated using a separate threshold for males and females, and age corrections to the thresholds) by gender and zygosity group
| Pairwise concordance rate | Correlation, r (95% CI) | |||
|---|---|---|---|---|
| Gender—zygosity group (twin pairs, n) | Depression | D-probe | Depression | D-probe |
| MZ—MM (360) | 0.163 | 0.242 | 0.27 (-0.08 to 0.57) | 0.25 (-0.01 to 0.48) |
| DZ—MM (261) | 0.182 | 0.257 | 0.28 (-0.06 to 0.57) | 0.29 (0.01 to 0.53) |
| MZ—FF (465) | 0.435 | 0.483 | 0.58 (0.41 to 0.72) | 0.63 (0.48 to 0.75) |
| DZ—FF (307) | 0.253 | 0.294 | 0.29 (0.02 to 0.53) | 0.30 (0.06 to 0.51) |
| DZ—OG (528) | 0.242 | 0.256 | 0.28 (0.08 to 0.47) | 0.17 (0.00 to 0.34) |
The low male prevalence and low male concordance for depression was reflected in just four MZ male pairs and four DZ male pairs concordant for depression. The low frequency of concordant-affected males would lead to uncertain conclusions if we were to run a twin model. Therefore we present a full gender-limitation twin model for D-probe, but the twin model for depression includes only women.
Model fit
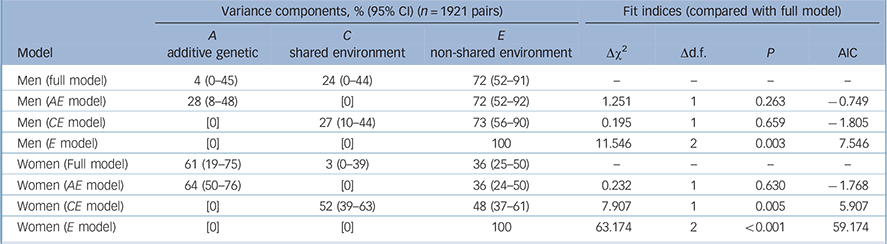
The fit of the full genetic model to the data can be performed only in the five-group analysis (D-probe), not the two-group depression analysis, because the latter model is ‘just identified’. The five-group model was a good fit to the data (Table 3, Δχ2 = 0.120, d.f. = 1, P = 0.729).
Table 3 ACE model fitting results and parameter estimates for D-probe (examining all five gender × zygosity groups)a
| Variance components, % (95% CI) (n = 1921 pairs) | Fit indices (compared with full model) | ||||||
|---|---|---|---|---|---|---|---|
| Model | A additive genetic | C shared environment | E non-shared environment | Δχ2 | Δd.f. | P | AIC |
| Men (full model) | 4 (0-45) | 24 (0-44) | 72 (52-91) | — | — | — | — |
| Men (AE model) | 28 (8-48) | [0] | 72 (52-92) | 1.251 | 1 | 0.263 | -0.749 |
| Men (CE model) | [0] | 27 (10-44) | 73 (56-90) | 0.195 | 1 | 0.659 | -1.805 |
| Men (E model) | [0] | [0] | 100 | 11.546 | 2 | 0.003 | 7.546 |
| Women (Full model) | 61 (19-75) | 3 (0-39) | 36 (25-50) | — | — | — | — |
| Women (AE model) | 64 (50-76) | [0] | 36 (24-50) | 0.232 | 1 | 0.630 | -1.768 |
| Women (CE model) | [0] | 52 (39-63) | 48 (37-61) | 7.907 | 1 | 0.005 | 5.907 |
| Women (E model) | [0] | [0] | 100 | 63.174 | 2 | <0.001 | 59.174 |
The statistical significance of the linear and quadratic regression coefficients of the threshold model age correction were tested. Both regression coefficients were significant (at P<0.05) in all models tested (i.e. for males and females in the D-probe model, and for females in the depression model), and so were retained in later models that estimated the A, C and E parameters. In the D-probe model, the regression coefficients could not be equated across gender (P < 0.05 for both the linear and quadratic components tested separately). However, if both the linear and the quadratic components were equated across gender at once, then there was no significant drop in fit (Δχ2 = 4.314, d.f. = 2, P = 0.116). Thus, we fitted genetic models using separate regression coefficients for men and women, but we noted that the age correction is similar across gender.
D-probe
There was no evidence of qualitative gender differences. However, there were significant quantitative gender differences (equating parameters representing A, C and E resulted in: Δχ2 = 9.488, d.f. = 2, P = 0.009). When testing the significance of the A and C parameters in the male and female models separately, the AE model was found to be the best fitting model for females (i.e., dropping C: P = 0.630). In the full model, genetic factors influenced 61% of the liability to depression, with the remainder influenced by non-shared environmental influences (Table 3).
In the male model, the χ2-test could not distinguish between the AE (i.e. dropping C: P = 0.263, Δ Akaike's information criterion (AIC) = −0.749) or CE (i.e. dropping A: P = 0.659, ΔAIC = −1.805) models as a better fit, although the CE model was a marginally better fit as indicated by a lower AIC value; A and C could not both be dropped, indicating significant familial influences on D-probe (P = 0.003) (Table 3). This inability to distinguish the source of the familial resemblance was despite having over 80% power to detect a genetic effect on depression in males, based on the effect size found in females. In addition, the estimates of non-shared environmental influence in the full ACE models had non-overlapping confidence intervals in men and women (men: 72%, CI 52–91%; women: 36%, CI 25–50%).
The age effects on D-probe were slightly greater among men and could not be equated across gender without worsening of fit (linear coefficient Δχ2 = 3.969, d.f. = 1, P = 0.046; quadratic coefficient Δχ2 = 4.003, d.f. = 1, P = 0.045).
Depression (female–female pairs only)
The variance in depression accounted for by genetic and environmental factors closely resembled the results for the broader D-probe definition in females, with 59% of the variance accounted for by genetic factors, and none accounted for by shared environmental factors (Table 4). The best fitting model was the AE model, since the C parameter could be dropped without worsening of fit; the CE model was a significantly worse fit (P = 0.036).
Table 4 ACE model fitting results and parameter estimates for depression (monozygotic female and dizygotic female groups only)a

| Variance components, % (95% CI) (n = 772 pairs) | Fit indices (compared to full model) | ||||||
|---|---|---|---|---|---|---|---|
| Model | A additive genetic | C shared environment | E non-shared environment | Δχ2 | Δd.f. | P | AIC |
| Women (full model) | 59 (4-72) | 0 (0-48) | 41 (28-57) | — | — | — | — |
| Women (AE model) | 59 (43-72) | [0] | 41 (28-57) | 0.00 | 1 | 1.00 | -2.000 |
| Women (CE model) | [0] | 49 (35-61) | 51 (39-65) | 4.419 | 1 | 0.036 | 2.419 |
| Women (E model) | [0] | [0] | 100 | 44.497 | 2 | <0.001 | 40.497 |
Effect of age correction of thresholds
If not accounted for, age might mimic the effect of C since it is correlated perfectly within twin pairs. The full ACE models were re-run with the age correction parameters set to zero, to examine the amount of error avoided in the parameter estimates by including an age correction. The percentage of the variance accounted for by the C parameter was estimated at 25, 3 and 0, and the proportion of the variance accounted for by the A factor was 6, 62 and 61 respectively in the male and female models for D-probe, and the female model for depression. Therefore, although not accounting for age generally led to incorrectly inflated C and heritability estimates, the change in magnitude was very small (0–2% of the total variance in liability).
Discussion
This is the first population-based twin study in a low-income country to assess the heritability of depressive disorder. We identified a true population sample of twins and achieved an exceptionally high participation rate (>90%), making our findings likely to be highly generalisable. We took special care to translate the study interview to take account of cultural meanings of depression and related concepts. Despite a large sample size, the relatively low lifetime prevalence of major depression means that we had limited statistical power particularly in men.
We have found that the aetiology of depression in this Sri Lankan context (in terms of the relative contribution of genes and environments) may differ in some respects compared with previous reports from higher-income, Western countries. The heritabilities of liability to depression and to a broader category, D-probe (two weeks of depressed mood or loss of interest), were similar to international estimates among women. The heritability of D-probe was significantly lower (and so non-shared environments contributed more) for men compared with women within this population. This heritability among Sri Lankan men appears considerably lower than in most studies from Europe, Australia and North America; however, this estimate for heritability among men is imprecise and the confidence intervals do still fall within the bounds of estimates shown in European-ancestry populations. Gender differences in depression heritability have inconsistently been reported in other countries, Reference Sullivan, Neale and Kendler1 and where found these have been in the same direction but of smaller magnitude than the present study. Reference Kendler, Gardner, Neale and Prescott2,Reference Kendler, Gatz, Gardner and Pedersen3 However, a gender difference in the same direction as the present study was found in a recent investigation of depressive symptoms in a South Korean volunteer twin sample. Reference Hur23
Comparisons of aetiology cross-nationally rely on the detected phenotypes being sufficiently similar. Impacts of confounding factors that may differ cross-nationally should also be considered. This sample had a fairly young age distribution (mean age of 34 years). Differences may have arisen through the way in which participants interpreted psychiatric questions, and their willingness to report symptoms in the face of stigma and cultural interpretations of symptoms of distress. Reference Goldney, Fisher and Hawthorne24,Reference Hsu, Davies and Hansen25 Countering these concerns, the episodes of depression we found Reference Ball, Siribaddana, Kovas, Glozier, McGuffin and Sumathipala15 consisted of a similar pattern of particular symptoms, total number of symptoms and sociodemographic correlates as has been found in other countries using full DSM–IV diagnostic criteria. Reference Kornstein, Schatzberg, Thase, Yonkers, McCullough and Keitner26,Reference Middeldorp, Wray, Andrews, Martin and Boomsma27 This suggests that the same underlying phenotype has been tapped into, rather than a subtype of depression that is substantially qualitatively different from that seen in other countries.
What can we infer from cross-national heritability comparisons?
Population genetic evidence suggests that most worldwide genetic variance in humans is seen within populations and only a small percentage of variance exists between populations or between countries. Reference Goldstein and Chikhi28 This implies that much of the genetic influence contributing to heritability estimates will be similar across countries. Therefore it is likely that any heritability differences we see across countries are because of differences in environments, or the extent to which genes co-act and interact with these. Although use of Western definitions of illness may be problematic in non-Western settings, Reference Summerfield29 there is remarkable similarity in symptom and environmental risk-factor profiles between populations. Reference Patel, Araya, de Lima, Ludermir and Todd30,Reference Simon, Goldberg, Von and Ustun31 However, the frequency of risk factors such as poverty and low educational status may differ between low- and middle-income and high-income countries. Comparing the aetiology results uncovered in the current study with results from a range of higher-income and non-Asian countries is thus likely to be informing us mostly about environmental rather than genetic differences across countries.
The current results are not necessarily generalisable to the whole island of Sri Lanka, in particular because we only sampled in one district that includes the capital city and neighbouring areas, and because the sample was predominantly of Sinhala ethnicity. Nonetheless, we gained a participation rate over 90% and this is the first large population-based twin study with a mental health focus in a non-Western country. This is important because heritability is a population-specific statistic: we may learn much about the fundamental aetiology of disorders such as depression by examining the relative influence of genes and environments in substantially different contexts.
We found that women's genetic liability to depression in Sri Lanka was if anything slightly higher (although with wide confidence intervals) than that reported in community samples in Western high-income countries. This suggests that the aetiology of female depression is similar across countries of different income levels, countering the hypothesis that greater variation in environmental exposures in Sri Lanka would produce a low heritability of depression. An alternative interpretation is that environmental factors with high variance in Sri Lanka (such as relative poverty) may not be having a large causal effect on female depression. This suggests that the environments that do contribute to female depression comprise factors that have a similar variance (or even a lower variance) in Sri Lanka compared with the West. It is also possible that qualitatively different environmental factors play a role in different countries, and operate through different mechanisms. The Sri Lankan cultural context may exert a blanket effect on women (i.e. an effect that influences most women, therefore with low variance) that emphasises the genetic component of depression, as compared with the Western world. As a speculative example, fewer opportunities for female emancipation in the Sri Lankan context may mean that many women are stuck in unhappy situations. However, those women who perform well at school (partly because of genetically inherited ability) may have a greater influence on the home situation they end up in later in life, thereby influencing their exposure to depressogenic situations. Consequently, a pervasive environmental exposure would not be identified as a shared environmental factor when examining one population using the twin design (because there is no variation in exposure in the population), but could act to increase heritability as measured within that population.
The lower prevalence of depressive disorders in men reduces the power to distinguish between genetic and environmental components of familial similarity, although depression in men was found to be significantly less heritable than in women. In addition, non-shared environmental influences were greater in men than in women. One explanation of the lower male heritability (and greater E) is a lower reliability of depression reporting among men in Sri Lanka, mimicking greater environmental variation, although there is inconclusive evidence for gender differences in reliability of depression reporting. Reference Foley, Neale and Kendler32,Reference Wells and Horwood33 There appeared to be a slightly greater effect of age on the prevalence of depression in men than in women, which might reflect lower willingness to report depression, or greater salience of certain aetiological risk factors among men of a particular age. The symptom profiles of depressive phenotypes in this sample appear similar across gender, Reference Ball, Siribaddana, Kovas, Glozier, McGuffin and Sumathipala15 suggesting that the same disorder is being detected among men and women. Thus the episodes detected are likely to be equally reliable in both genders, although it is still possible for low reliability to exist as one gender is less likely to report any of their symptoms.
On the other hand, it is possible that certain ‘real’ environmental factors play an important role for the development of depression in men only. For example, certain aspects of standards of living, employment patterns and residence in a more urbanised area appear to be more strongly associated with depression in males than in females in this sample. Reference Ball, Siribaddana, Kovas, Glozier, McGuffin and Sumathipala15
Limitations
All classic twin studies, including the current study, are based on the equal environments assumption, i.e. that the degree of environmental similarity is constant across zygosities. This assumption has been tested and supported in relation to its impact on affective disorders, including depression. Reference Kendler, Neale, Kessler, Heath and Eaves34,Reference Kendler and Prescott35
The low prevalence of the phenotypes in men led to wide confidence intervals in the genetic model. The upper end of the interval for the male D-probe model does in fact overlap with findings from higher-income, Western countries, but the pattern nonetheless is most consistent with a low heritability in men. One of the most striking findings is the magnitude of the gender difference in the genetic models, which has not been seen in higher-income countries.
In the current study our assessment of depression did not include a requirement for functional impairment, and the D-probe phenotype only required one of two core symptoms of a depressive episode. A low prevalence of depressive episodes that met full DSM criteria reduced power to run genetic models. However, the less stringent phenotypes that we identified do appear to be on the same diagnostic continuum as the full diagnosis (in terms of having similar symptom profiles and sociodemographic associations). Also, the requirement for functional impairment may not be as relevant in this population as for those for which it was designed. Reference Ball, Siribaddana, Kovas, Glozier, McGuffin and Sumathipala15 The liability threshold model of disorders assumes that everyone in a population is to a greater or lesser degree liable to develop a disorder, with diagnostic thresholds identifying those above a certain level of liability. Under this assumption, the current study is likely to have tapped into the same dimension as if we had been able to fully analyse a more stringent definition of depression.
In conclusion, these results from a large twin sample in a low/middle-income country suggest that different aetiological processes may be at work in this Sri Lankan setting relative to previously studied countries. As well as highlighting processes particular to Sri Lanka, these differences can inform our general understanding of the aetiology of depression throughout the world.
Funding
The Wellcome Trust provided funding for the CoTASS study (grant no. 069629/Z/02/A), and the Institute for Research and Development, Sri Lanka, provided infrastructural support. H.B. was supported by an ESRC research studentship. M.H. is funded by the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King's College London, National Institute of Health Research, Biomedical Research Centre.









eLetters
No eLetters have been published for this article.