Tobacco dependence is one of the major causes of premature death and disability in the world. 1 People with schizophrenia have three times the odds of ever smoking than the general population and their smoking cessation rates are lower than the smoking cessation rates in the general population. Reference de Leon and Diaz2 Furthermore, smokers with schizophrenia may smoke more heavily and extract more nicotine from each cigarette. Reference Olincy, Young and Freedman3–Reference Williams, Ziedonis, Abanyie, Steinberg, Foulds and Benowitz5 Tobacco may also be used to alleviate some of the symptoms in schizophrenia and the side-effects of antipsychotic medications. Reference Adler, Hoffer, Wiser and Freedman6,Reference Sacco, Bannon and George7 Using nicotine to ‘self-medicate’, together with depressive symptoms, drug misuse, disorganised thinking and poor task persistence in people with schizophrenia, may explain the lower motivation and the greater difficulty for smoking cessation in these individuals. Reference Addington, el-Guebaly, Addington and Hodgins8 Because of the particular set of challenges they face in attempting to quit smoking, there is a need to investigate effective treatments for smoking cessation in people with schizophrenia. There is robust evidence supporting bupropion as a safe and effective treatment for nicotine dependence in the general population. Reference Hughes, Stead and Lancaster9 Bupropion is an atypical antidepressant with both dopaminergic and adrenergic actions. Reference Stahl, Pradko, Haight, Modell, Rockett and Learned-Coughlin10 Research suggests that bupropion acts as a non-competitive antagonist at the nicotinic acetycholinergic receptor. Reference Fryer and Lukas11 Bupropion may also have an effect on the brain reward system, which may contribute to its action on smoking cessation. Reference Cryan, Bruijnzeel, Skjei and Markou12 However, the benefits and harms of bupropion as an aid for smoking cessation in schizophrenia remain uncertain. Smokers with schizophrenia have a more severe nicotine dependence compared with other smokers, and bupropion is metabolised by the cytochrome P450 system in the liver and it may interact with the commonly used antipsychotic medications metabolised by the same system. This, as well as bupropion's dopaminergic action, may adversely affect the mental state of individuals with schizophrenia. These uncertainties are reflected in treatment guidelines for smoking cessation in schizophrenia. In one guideline, the use of bupropion as a drug treatment for tobacco dependence was not recommended, as there was concern that bupropion may precipitate or exacerbate a psychotic relapse because of its pharmacodynamic and pharmacokinetic properties. Reference Strasser13
There is therefore a strong need for evidence regarding effective smoking cessation treatments for people with schizophrenia. There is evidence of benefit and few harms of bupropion in the general population, but there is uncertainty whether this benefit is generalisable and there are specific safety concerns for use of bupropion among individuals with schizophrenia. Thus, we aimed to systematically review and synthesise all current evidence of efficacy and safety of bupropion as a treatment for nicotine dependence in individuals with schizophrenia.
Method
Inclusion criteria
All randomised controlled trials (RCTs) comparing bupropion with placebo or with a different therapeutic control in adult smokers with a current diagnosis of schizophrenia according to either the ICD–10 14 or the DSM–IV 15 were included. Trials using bupropion together with other co-interventions (either pharmacological or non-pharmacological) were also included and the trial control arms could be pharmacological or non-pharmacological or both. Trials that used bupropion for purposes other than smoking cessation or reduction, as well as trials which investigated people with other psychiatric diagnoses were excluded. However, we included studies of individuals with schizophrenia who had substance use disorders in addition to nicotine dependence, as the prevalence of these disorders among people with schizophrenia can be substantial. Reference Dixon16
Data sources and search strategy
One of the authors (D.T.T.) searched the Cochrane Central Register of Controlled Trials (CENTRAL) of the Cochrane Library, MEDLINE, EMBASE and PsycINFO from inception to 7 March 2009. To locate unpublished studies, conference abstracts, records of trials held by the manufacturers of bupropion GlaxoSmithKline (www.gsk-clinicalstudyregister.com/) and reference lists of all relevant studies were searched. Full details of the search strategy are listed in the online supplement.
Outcome measures
The following outcome measures were included.
-
(a) Abstinence from smoking at the end of bupropion treatment. Abstinence was measured either by self-report or biochemical verification (such as expired carbon monoxide (CO) level) or both. In each study, we used the strictest available criteria to define abstinence. For instance, if both sustained abstinence and point-prevalence abstinence rates were presented, data for sustained abstinence were extracted in preference to point prevalence. In studies that used biochemical validation of abstinence, only those participants who met the criteria of cessation biochemically were classified as abstinent.
-
(b) Change in severity of smoking dependence at the end of bupropion treatment (reduction). Change in severity of smoking dependence was measured by change in expired CO level, self-reported reduction in number of cigarettes smoked or other biochemical measurement.
-
(c) Change in the mental state at the end of bupropion treatment, measured by change in positive, negative and depressive symptoms using validated measurement tools.
We also included abstinence and change in severity of smoking dependence after 6 months follow-up from the start of bupropion therapy. West et al suggested some common standards (the ‘Russell Standard’) for outcome criteria in smoking cessation trials including duration and definition of abstinence, the need for biochemical verification, use of intention-to-treat analysis, the way to handle protocol violators and the importance of masking. Reference West, Hajek, Stead and Stapleton17 We chose 6 months because the Russell Standard states that a sustained period of abstinence for at least 6 months is required to provide confidence that abstinence will continue long term and that a degree of health benefit will be achieved. A report by the US Department of Health and Human Services also suggests that measuring abstinence at less than 6 months does not provide an accurate prediction of long-term cessation. Reference Fiore, Jaén, Baker, Bailey, Benowitz and Curry18 The US Department of Health and Human Services Tobacco Use and Dependence Guideline Panel also suggested a minimum 6-month period as an adequate period of abstinence to assess treatment differences in the longer term, as a brief period of abstinence is unlikely to have a significant health benefit. Any adverse events reported in each trial were also recorded and assessed.
Data extraction and study appraisal
Two authors (D.T.T. and A.C.W.) independently examined the search results and screened the titles and abstracts, and subsequently the full text reports of all the potentially relevant trials. Two authors (D.T.T. and M.P.) independently abstracted data from all the included trials using a standardised form. Trial methodological quality was also assessed over four domains: concealment of allocation; masking; completeness of follow-up; and use of intention-to-treat analysis. Any differences of opinion were resolved by discussions among all three authors. When necessary, we attempted to contact report authors to clarify uncertainties in the study design and the results or possible duplicate reporting of the same patient group.
Data synthesis
One author (D.T.T.) used Review Manager (RevMan) 5.0.17 for Windows (Cochrane Collaboration; see www.cc-ims.net/revman) to synthesise summary estimates. Results for dichotomous outcomes were calculated using the Mantel–Haenszel method and expressed as risk ratio (RR) with 95% confidence intervals, where risk ratio greater than 1.0 favours bupropion treatment. Results for continuous outcomes were expressed as mean difference where measured with the same scale, or standardised mean difference where measured with different scales, calculated using the inverse variance approach, with 95% confidence intervals. A summary mean difference or standardised mean difference below zero favoured bupropion treatment. We used random effects analysis as this gives a more conservative estimate in the presence of potential heterogeneity. Heterogeneity among trials was estimated using both Cochran Q test and I 2 statistic. Reference Higgins, Thompson, Deeks and Altman19
Subgroup analyses were performed to compare trials where bupropion was used as a single intervention with trials where bupropion was used as an adjunct to other drug therapy, such as nicotine replacement therapy (NRT). Sensitivity analyses were also conducted to assess the effect of different publication types (e.g. a full journal article v. a conference abstract) and different dosages of bupropion used.
Results
Literature search, characteristic and quality of included studies
We identified and included 21 reports of 7 trials with a total of 260 participants. The process of trial identification is summarised in Fig. 1, and the characteristics of all included trials in Table 1. All the reports were in English and all the trials were conducted in the USA, with participants recruited from the community. All studies except one Reference Fatemi, Stary, Hatsukami and Murphy20 included people who expressed a desire to quit smoking and were willing to set a target quit date. Individuals with active substance misuse in addition to nicotine were excluded in all the trials apart from one. Reference Fatemi, Stary, Hatsukami and Murphy20
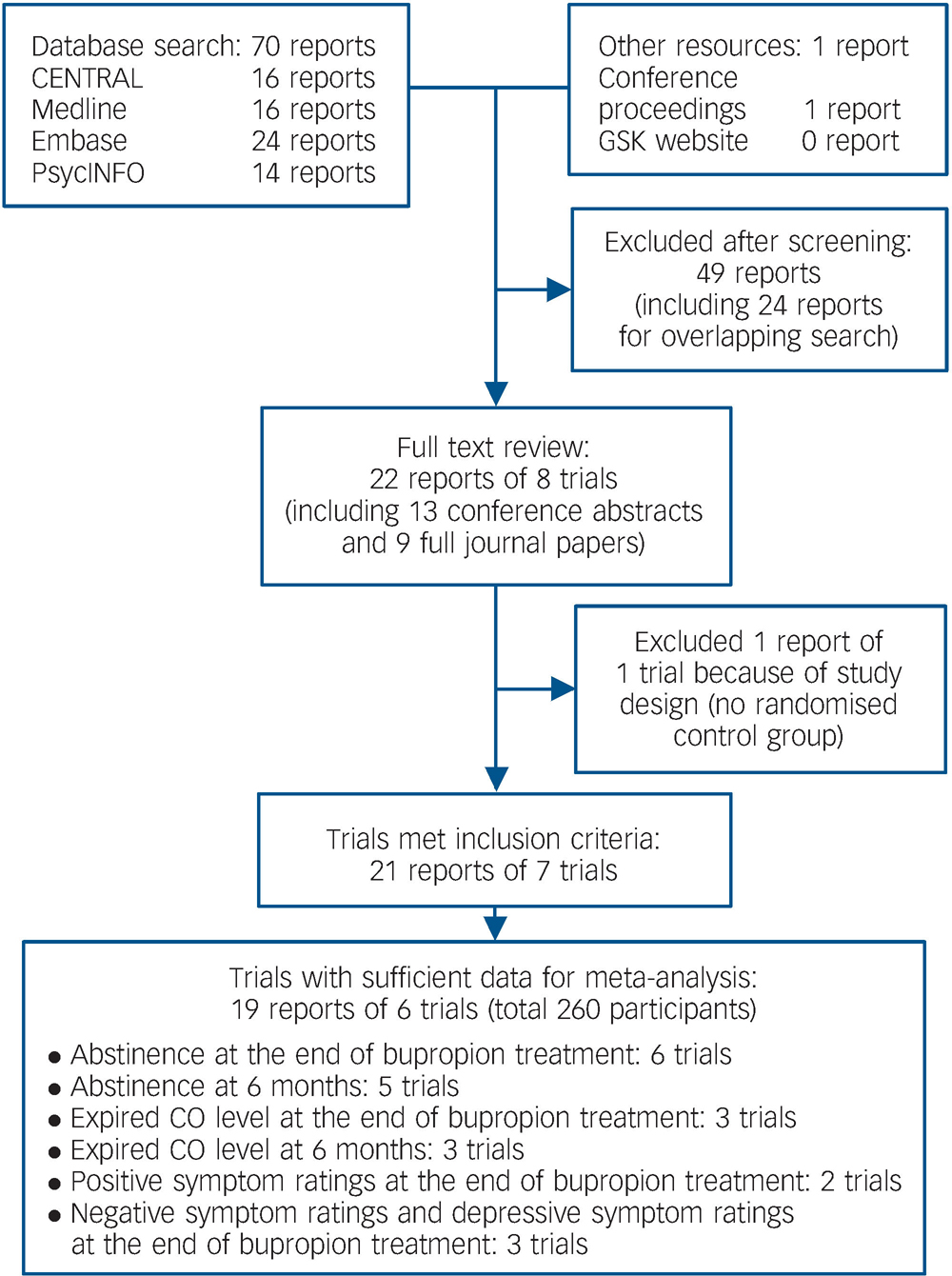
Fig. 1 Summary of the process of identifying randomised trials for inclusion in this systematic review of bupropion for smoking cessation in people with schizophrenia. CO, carbon monoxide.
Table 1 Characteristics of trials included in this systematic review of bupropion for smoking cessation in people with schizophrenia

| Trials | Setting | Participants, n | Dose and duration of bupropion | Co-interventions | Abstinence verification | Length of follow-up |
|---|---|---|---|---|---|---|
| Evins (2001) Reference Evins, Mays, Rigotti, Tisdale, Cather and Goff32,Reference Evins, Cather, Goff and Rigotti34,Reference Evins, Cather, Rigotti, Freudenreich, Henderson and Olm-Shipman36 | Single centre | 19 | 150 mg daily for 12 weeks | Hourly group CBT for 9 weeks | Expired CO level < 9ppm or serum cotinine < 14 ng/ml | 2 years |
| George (2002) Reference George, Hitsman, Papandonatos, Sacco, Vessicchio and Dudas21,Reference Sacco, Hitsman, Papandonatos, Vessicchio, Dudas and Termine26,Reference George, Vessicchio, Termine, Bregartner, Feingold and Rounsaville33,Reference Vessicchio, Termine, Bregartner and George35 | Single centre | 32 | 150 mg daily for first 3 days and then 300 mg daily for 10 weeks | Hourly group therapy (motivation enhancement, psychoeducation and relapse prevention) for 10 weeks | Expired CO level < 10 ppm | 6 months |
| Evins (2005) Reference Evins, Cather, Deckersbach, Freudenreich, Culhane and Olm-Shipman28-Reference Evins, Cather, Goff, Olm-Shipman and Rigotti31 | Multi-centre | 53 | 150 mg daily for first week and 300 mg daily for 12 weeks | Hourly group CBT for 12 weeks | Expired CO level < 9 ppm | 24 weeks |
| Fatemia (2005) Reference Fatemi, Stary, Hatsukami and Murphy20 | Single centre | 10 | Unclear | Not mentioned | Monitored expired CO level but unclear definition of abstinence | Cross-over design: 3 weeks bupropion/placebo, 1 week washout and 3 week placebo/bupropion |
| Evins (2007) Reference Evins, Cather, Culhane, Birnbaum, Horowitz and Hsieh22,Reference Evins, Cather, Culhane, Birnbaum, Horowitz and Hsieh24 | Multi-centre | 51 | 150 mg daily for first week and 300 mg daily for 12 weeks | Nicotine patch from week 4 (21 mg/day for 4 weeks, then 14 mg/day for 2 weeks, 7 mg/day for another 2 weeks) + as required nicotine gum + hourly group CBT for 12 weeks | Expired CO level ≤ 8 ppm | 6 months |
| George (2008) Reference George, Hitsman, Papandonatos, Sacco, Vessicchio and Dudas21,Reference George, Vessicchio, Sacco, Weinberger, Dudas and Allen23,Reference George, Vessicchio, Weinberger and Sacco25-Reference George, Vessicchio, Allen, Weinberger and Sacco27 | Single centre | 59 | 150 mg daily for 3 days and then 300 mg daily for 10 weeks | TNP (21 mg/24 h) + group behavioural therapy for 10 weeks (50 min each week) | Expired CO level < 10 ppm | 6 months |
| Weiner (2007) Reference Weiner, Buchanan, Gold, Ball and Bennett37,Reference Weiner, Ball, Buchanan and Gold38 | Multi-centre | 46 | 300 mg daily for 12 weeks (week 2 to week 14) | Group therapy for 9 weeks | Expired CO level < 10 ppm | 14 weeks |
Two trials used bupropion as an adjunct to NRT, Reference George, Hitsman, Papandonatos, Sacco, Vessicchio and Dudas21–Reference George, Vessicchio, Allen, Weinberger and Sacco27 whereas the other trials used bupropion as a single-agent drug therapy for smoking cessation. One trial employed a cross-over design and participants received either bupropion or placebo for 3 weeks, then a week without intervention as a washout period, followed by cross-over to the alternative regimen for 3 more weeks. Reference Fatemi, Stary, Hatsukami and Murphy20 All trials except one included group psychological intervention in addition to drug treatment. Reference Fatemi, Stary, Hatsukami and Murphy20 All trials included a measurement of expired CO level as biological validation of participants' self–report. Five trials reported follow-up data for at least 6 months from the start of bupropion treatment. Reference George, Hitsman, Papandonatos, Sacco, Vessicchio and Dudas21–Reference George, Vessicchio, Sacco, Weinberger, Dudas and Allen23,Reference Sacco, Hitsman, Papandonatos, Vessicchio, Dudas and Termine26,Reference Evins, Cather, Deckersbach, Freudenreich, Culhane and Olm-Shipman28–Reference Evins, Cather, Rigotti, Freudenreich, Henderson and Olm-Shipman36 One trial reported only as conference proceedings. Reference Weiner, Buchanan, Gold, Ball and Bennett37,Reference Weiner, Ball, Buchanan and Gold38 All studies reported monitoring of mental state before and after bupropion treatment.
Table 2 summaries the methodological quality of these studies regarding masking, completeness of follow-up and use of intention-to-treat analysis. No trial gave details of the method of randomisation or allocation concealment in their reports and we did not receive any clarification from the authors before we submitted this review. The reports of three studies stated that intention-to-treat analysis was used. However, participants who were randomised but dropped out before receiving the study intervention in these three trials were not included in the analysis. Reference George, Vessicchio, Sacco, Weinberger, Dudas and Allen23,Reference Evins, Cather, Deckersbach, Freudenreich, Culhane and Olm-Shipman28,Reference Evins, Mays, Rigotti, Tisdale, Cather and Goff32 Four trials explicitly report checking participants' adherence to taking their medication. Placebo was matched in appearance with the bupropion tablet in four studies.
Table 2 Summary of methodological quality indicators of trials included in this systematic review of bupropion for smoking cessation in people with schizophrenia
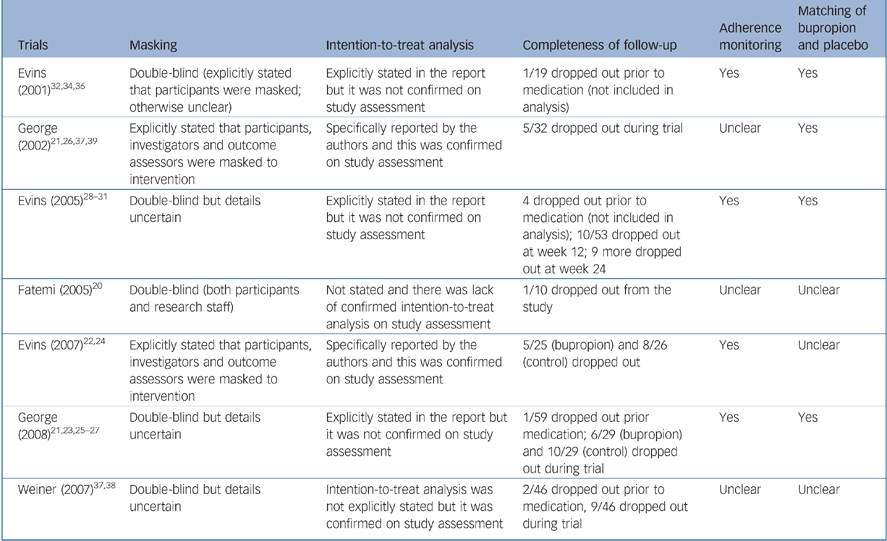
| Trials | Masking | Intention-to-treat analysis | Completeness of follow-up | Adherence monitoring | Matching of bupropion and placebo |
|---|---|---|---|---|---|
| Evins (2001) Reference Evins, Mays, Rigotti, Tisdale, Cather and Goff32,Reference Evins, Cather, Goff and Rigotti34,Reference Evins, Cather, Rigotti, Freudenreich, Henderson and Olm-Shipman36 | Double-blind (explicitly stated that participants were masked; otherwise unclear) | Explicitly stated in the report but it was not confirmed on study assessment | 1/19 dropped out prior to medication (not included in analysis) | Yes | Yes |
| George (2002) Reference George, Hitsman, Papandonatos, Sacco, Vessicchio and Dudas21,Reference Sacco, Hitsman, Papandonatos, Vessicchio, Dudas and Termine26,Reference Weiner, Buchanan, Gold, Ball and Bennett37,Reference Eliasson, Hjalmarson, Kruse, Landfeldt and Westin39 | Explicitly stated that participants, investigators and outcome assessors were masked to intervention | Specifically reported by the authors and this was confirmed on study assessment | 5/32 dropped out during trial | Unclear | Yes |
| Evins (2005) Reference Evins, Cather, Deckersbach, Freudenreich, Culhane and Olm-Shipman28-Reference Evins, Cather, Goff, Olm-Shipman and Rigotti31 | Double-blind but details uncertain | Explicitly stated in the report but it was not confirmed on study assessment | 4 dropped out prior to medication (not included in analysis); 10/53 dropped out at week 12; 9 more dropped out at week 24 | Yes | Yes |
| Fatemi (2005) Reference Fatemi, Stary, Hatsukami and Murphy20 | Double-blind (both participants and research staff) | Not stated and there was lack of confirmed intention-to-treat analysis on study assessment | 1/10 dropped out from the study | Unclear | Unclear |
| Evins (2007) Reference Evins, Cather, Culhane, Birnbaum, Horowitz and Hsieh22,Reference Evins, Cather, Culhane, Birnbaum, Horowitz and Hsieh24 | Explicitly stated that participants, investigators and outcome assessors were masked to intervention | Specifically reported by the authors and this was confirmed on study assessment | 5/25 (bupropion) and 8/26 (control) dropped out | Yes | Unclear |
| George (2008) Reference George, Hitsman, Papandonatos, Sacco, Vessicchio and Dudas21,Reference George, Vessicchio, Sacco, Weinberger, Dudas and Allen23,Reference George, Vessicchio, Weinberger and Sacco25-Reference George, Vessicchio, Allen, Weinberger and Sacco27 | Double-blind but details uncertain | Explicitly stated in the report but it was not confirmed on study assessment | 1/59 dropped out prior medication; 6/29 (bupropion) and 10/29 (control) dropped out during trial | Yes | Yes |
| Weiner (2007) Reference Weiner, Buchanan, Gold, Ball and Bennett37,Reference Weiner, Ball, Buchanan and Gold38 | Double-blind but details uncertain | Intention-to-treat analysis was not explicitly stated but it was confirmed on study assessment | 2/46 dropped out prior to medication, 9/46 dropped out during trial | Unclear | Unclear |
Data synthesis and meta-analysis
Participants taking bupropion were two and a half times more likely to be abstinent at the end of treatment, compared with those on placebo (6 trials, 260 participants, RR = 2.57, 95% CI 1.35–4.88, heterogeneity P = 0.54, I2 = 0%) (Fig. 2). Subgroup analysis revealed that abstinence may be more likely when bupropion was combined with NRT (RR = 2.92, 95% CI 0.75–11.33), compared with using bupropion as the only pharmacotherapy (RR = 2.77, 95% CI 1.05–7.32). Sensitivity analysis showed an increased risk ratio when a trial reported only as a conference abstract Reference Weiner, Ball, Buchanan and Gold38 was removed from the analysis (RR = 3.02, 95% CI 1.45–6.32). Similar results were obtained when the trial Reference Evins, Mays, Rigotti, Tisdale, Cather and Goff32 using low dose (150 mg bupropion daily compared with 300 mg daily in other trials) was excluded (RR = 2.58, 95% CI 1.32–5.02).
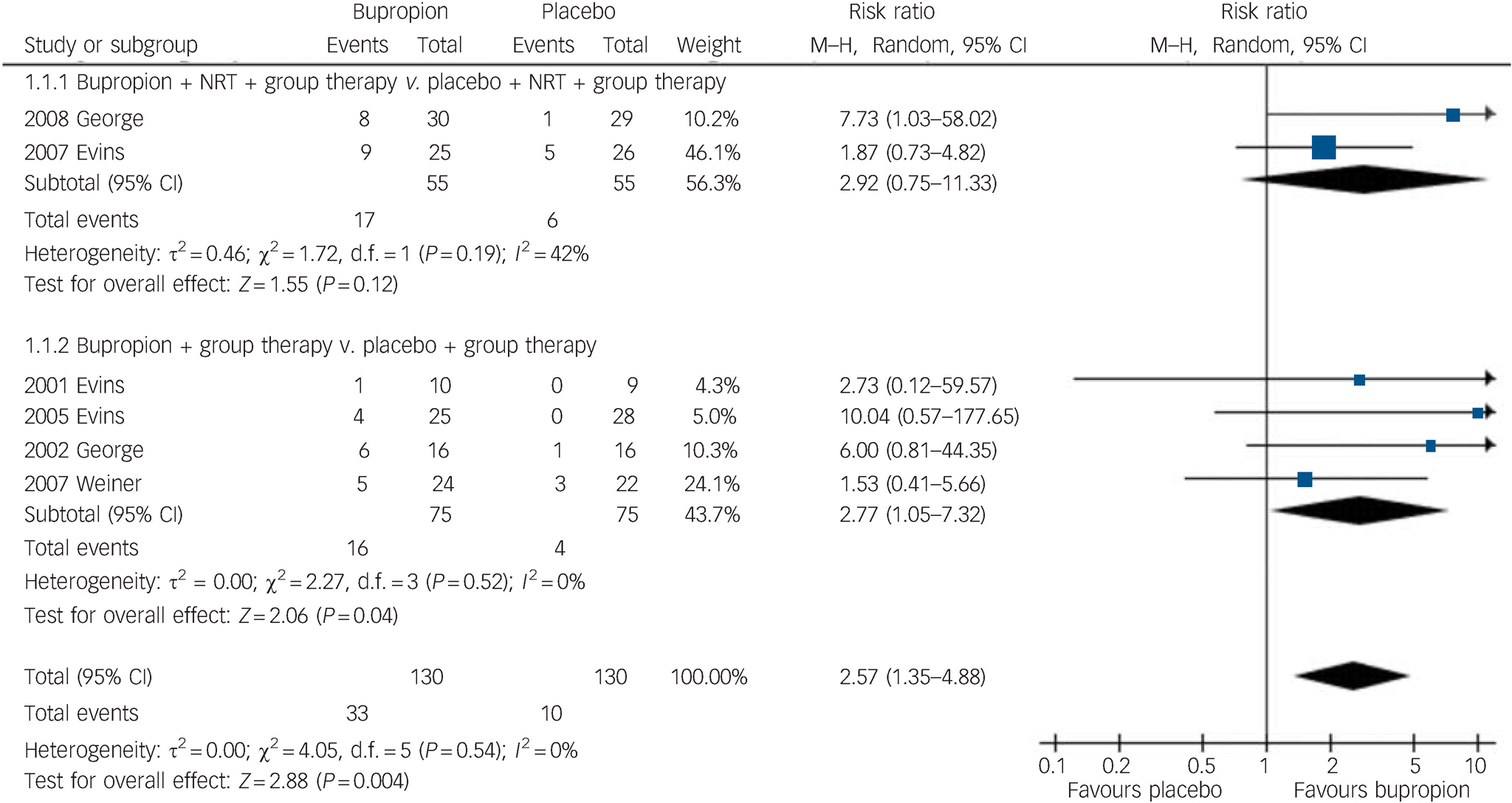
Fig. 2 Risk of smoking abstinence at the end of bupropion treatment compared with placebo. M–H, Mantel–Haenszel method; NRT, nicotine replacement therapy.
Abstinence was sustained and unchanged at 6 months after starting bupropion (5 trials, 214 participants, RR = 2.78, 95% CI 1.02–7.58) (Fig. 3). Subgroup analysis also suggested that maintained abstinence was more likely when bupropion was used together with NRT (2 trials, 110 participants, RR = 3.41, 95% CI 0.87–13.30) compared with using bupropion alone (3 trials, 104 participants, RR = 2.19, 95% CI 0.50–9.63).
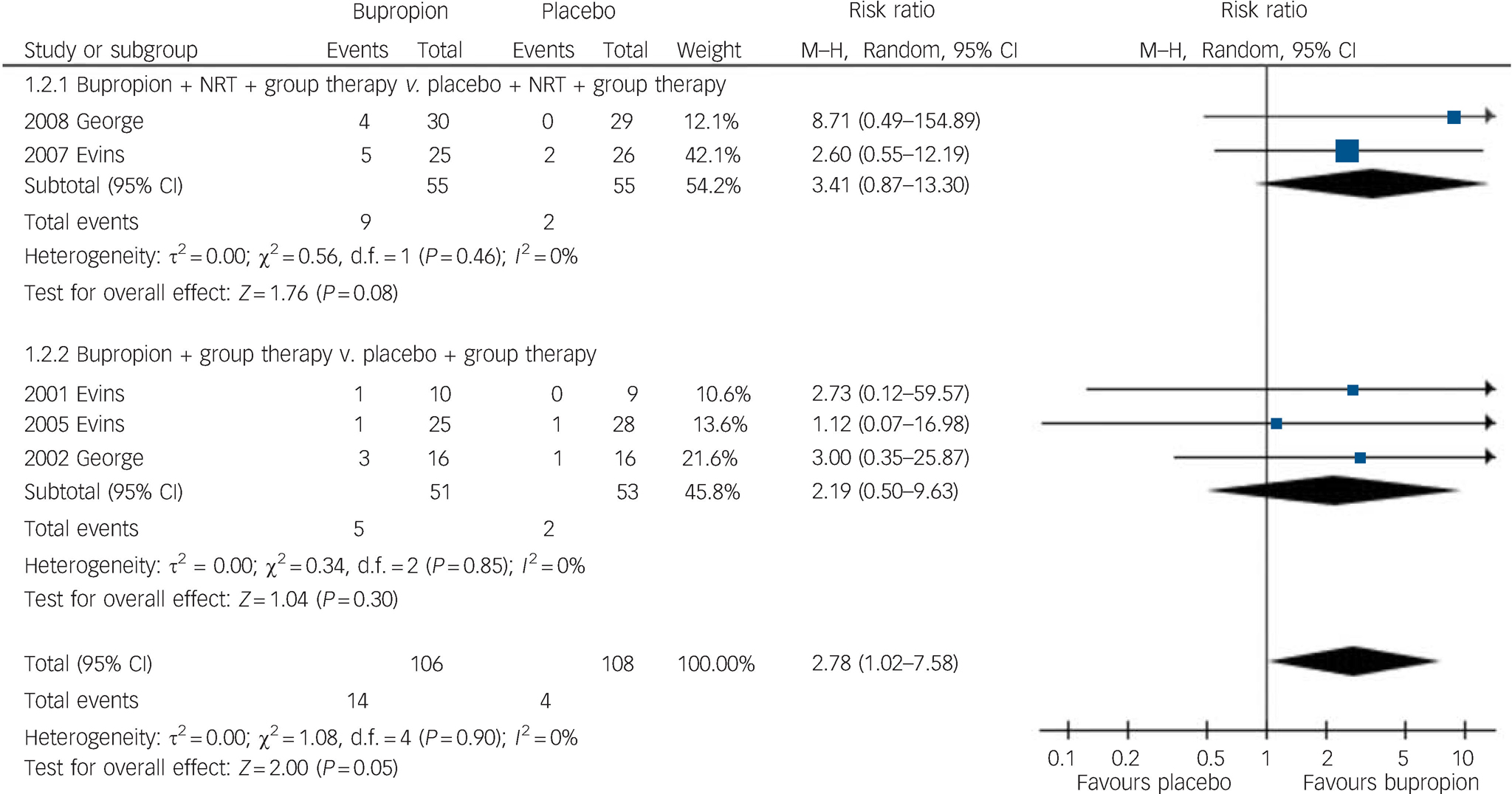
Fig. 3 Risk of smoking abstinence 6 months after start of bupropion compared with placebo. M–H, Mantel–Haenszel method; NRT, nicotine replacement therapy.
Six trials reported smoking reduction using expired CO level, but only three trials (123 participants) provided sufficient data for meta-analysis. Reference Evins, Cather, Culhane, Birnbaum, Horowitz and Hsieh22,Reference Evins, Cather, Deckersbach, Freudenreich, Culhane and Olm-Shipman28,Reference Evins, Mays, Rigotti, Tisdale, Cather and Goff32 At the end of bupropion treatment, there was a significant reduction in the expired CO level of individuals in the bupropion group compared with the control group (mean difference –6.84 parts per million (ppm), 95% CI –11.11 to –2.56 ppm, heterogeneity P = 0.44, I2 = 0%). However, at 6 months after the start of bupropion treatment there was no significant difference in the expired CO level (mean difference –5.73 ppm, 95% CI –18.09 to 6.63 ppm) but there was substantial heterogeneity among trials (P = 0.003, I2 = 83%), largely because one trial reported a higher expired CO level in the bupropion group at 6 months. Reference Evins, Cather, Deckersbach, Freudenreich, Culhane and Olm-Shipman28 The three trials reporting incomplete data for the expired CO level also favoured bupropion at the end of the treatment. Reference Fatemi, Stary, Hatsukami and Murphy20,Reference George, Vessicchio, Termine, Bregartner, Feingold and Rounsaville33,Reference Weiner, Ball, Buchanan and Gold38
All trials reported some measures of mental state before and after bupropion. Compared with placebo, bupropion did not cause any significant deterioration of positive, negative and depressive symptoms in people with schizophrenia when it was used as an aid for smoking cessation. Two studies provided sufficient data for estimation of change of positive symptoms (85 participants) Reference Evins, Cather, Deckersbach, Freudenreich, Culhane and Olm-Shipman28,Reference George, Vessicchio, Termine, Bregartner, Feingold and Rounsaville33 and one additional study also provided sufficient data to estimate the effect of bupropion on negative and depressive symptoms (136 participants). Reference Evins, Cather, Culhane, Birnbaum, Horowitz and Hsieh22 There was no evidence of a difference for bupropion compared with control in positive symptoms (standardised mean difference –0.24, 95% CI –0.66 to 0.19), negative symptoms (standardised mean difference –0.12, 95% CI –0.46 to 0.22) or depressive symptoms (standardised mean difference –0.16, 95% CI –0.50 to 0.18).
Regarding other adverse effects of bupropion, no trials reported any seizures. The prevalence of dry mouth was significantly higher in the bupropion group compared with the control group in one study (P<0.05). Reference George, Vessicchio, Termine, Bregartner, Feingold and Rounsaville33 The same research group, in a second study, reported significant differences on concentration, jitteriness, light-headedness, muscle stiffness and frequent nocturnal awakening in the bupropion group. Reference George, Vessicchio, Sacco, Weinberger, Dudas and Allen23 Of 59 participants, 3 (2 in the placebo group and 1 in the bupropion group) had a psychotic breakdown during the trial, but the authors concluded these psychotic breakdowns were unrelated to bupropion. In another study, one participant who was randomised to bupropion also had an allergic reaction to the medication. Reference Evins, Cather, Deckersbach, Freudenreich, Culhane and Olm-Shipman28 In a different trial, two people on bupropion and NRT dropped out from the trial because of insomnia and dizziness. Reference Evins, Cather, Culhane, Birnbaum, Horowitz and Hsieh22 One trial did not mention any adverse effects in the reports Reference Weiner, Buchanan, Gold, Ball and Bennett37,Reference Weiner, Ball, Buchanan and Gold38 and the remaining trials either reported ‘no serious adverse events’ Reference Evins, Mays, Rigotti, Tisdale, Cather and Goff32 or ‘no indications of increased side effects’. Reference Fatemi, Stary, Hatsukami and Murphy20
Discussion
Smokers with schizophrenia who used bupropion to aid smoking cessation had a two and a half times higher rate of abstinence at the end of the drug therapy compared with those who did not use bupropion. Abstinence was sustained at 6 months after the treatment, even though all participants took the drug for 12 weeks or less, and the results were consistent among trials. There is no evidence from this meta-analysis to suggest that smokers with schizophrenia who used bupropion for smoking cessation differed significantly in positive, negative and depressive symptoms of schizophrenia from smokers on placebo. Although some side-effects of treatment that might be important to individuals were noted, there were no significant serious adverse clinical events such as seizure.
This is the first review showing that bupropion can increase the rate of smoking abstinence in smokers with schizophrenia. Only one study demonstrated a statistically significant increase in the abstinence rate with bupropion Reference George, Vessicchio, Sacco, Weinberger, Dudas and Allen23 and the other studies showed non-significant trends of the efficacy of bupropion in people with schizophrenia. This was likely as a result of the small sample size in each individual study. By pooling the data together in the meta-analysis, this systematic review revealed the beneficial effect of bupropion in smoking abstinence among individuals with schizophrenia.
In addition, at the end of treatment, the expired CO level in the bupropion group was 6.84 ppm lower than in the placebo group. Reduction of the expired CO level is a good indicator of smoking reduction and this result suggests that bupropion is also effective in reducing cigarette smoking. Evidence from this meta-analysis did not show a sustained effect at 6 months. However, only three trials provided sufficient data for meta-analysis and there was also a significant heterogeneity among trials for the expired CO level at 6 months.
Clinical implications
There is substantial evidence suggesting that bupropion is beneficial for smoking cessation in the general public. Reference Hughes, Stead and Lancaster9 Based on the best currently available evidence, the results of this systematic review support the effectiveness of bupropion in smoking cessation in individuals with schizophrenia. Smoking cessation and even reduction may have a significant effect on the reduction of cardiovascular risk. Reference Eliasson, Hjalmarson, Kruse, Landfeldt and Westin39,Reference Hatsukami, Kotlyar, Allen, Jensen, Li and Le40 Individuals with schizophrenia have a much higher risk of developing cardiovascular disease, which contributes to the increased rate of premature mortality compared with the general population. Reference Hennekens, Hennekens, Hollar and Casey41,Reference Osby, Correia, Brandt, Ekbom and Sparen42 There are other factors contributing to the elevated cardiovascular risk in this group of people. These include unhealthy diet, poor engagement with medical care and physical inactivity resulting in obesity. Reference Daumit, Goldberg, Anthony, Dickerson, Brown and Kreyenbuhl43,Reference Strassnig, Brar and Ganguli44 Increased prevalence of smoking among people with schizophrenia also contributes substantially to their higher cardiovascular risk. Reference McCreadie45 Furthermore, atypical antipsychotics can cause significant weight gain, glucose dysregulation and lipid abnormality, which can further raise the already high risk of cardiovascular disease. Reference Newcomer and Haupt46–Reference Marder, Essock, Miller, Buchanan, Casey and Davis48 Apart from cardiovascular diseases, chronic cigarette smoking in people with schizophrenia has also been reported as an important contributing factor to the higher morbidity and mortality from malignancy and respiratory diseases. Reference Brown, Inskip and Barraclough49,Reference Lichtermann, Ekelund, Pukkala, Tanskanen and Lonnqvist50
Stopping or reducing smoking in people with schizophrenia is a priority for healthcare professionals and for public health policy makers. Recent guidelines on smoking cessation have acknowledged this by including special guidelines for those with schizophrenia. Reference Fiore, Jaén, Baker, Bailey, Benowitz and Curry18,Reference Zwar, Richmond, Borland, Peters, Stilman and Litt51 Our review has shown that there is no evidence to support exacerbation of psychosis secondary to the use of bupropion in schizophrenia. In the general population, seizure is a recognised side-effect of bupropion, with a rate of about 1 in 1000. There have been some concerns among clinicians that the risk of seizure with bupropion may potentially be much higher for people with schizophrenia, as many antipsychotic medications are already well-known to increase the risk of developing seizure and co-intervention with bupropion may further exacerbate this risk. There was no report of seizure in any of the included trials. However, the total number of individuals randomised to bupropion was relatively small (130 participants). Hence, the evidence should be interpreted with caution, and the relationship between seizure and bupropion in schizophrenia needs further confirmation. Nevertheless, the results of this review can inform further guideline development and revisions for smoking cessation in schizophrenia, which is particularly important in the context of enforced smoking bans including mental health units in the UK. 52
Implications for future research
Although smokers with schizophrenia who used bupropion were more likely to abstain from smoking 6 months after the start of drug treatment, more research is necessary to examine whether abstinence can be maintained over a prolonged period. Only one study followed patients for 2 years after bupropion intervention. In that study, people with schizophrenia who reduced smoking significantly after bupropion were more likely to abstain from smoking after 2 years. Reference Evins, Cather, Rigotti, Freudenreich, Henderson and Olm-Shipman36 In addition, the sample size of each study was also relatively small (the largest sample size was 30 in one arm). Using the crude abstinence rate of about 8% in the placebo group and the meta-analytical risk ratio of 2.57 from this review of cessing smoking after taking bupropion, the calculated required minimum sample size for a trial of 5% statistically significance and 80% power is 136 (calculation according to Statcalc in EpiInfo version 3.5.1 for Windows (Centers for Disease Control and Prevention, Atlanta, see www.cdc.gov/epiinfo/epiinfo.htm)). Hence, future study design should focus on a larger sample size as well as a longer follow-up such as a year. Collaboration between multiple research centres may be necessary because of the potential difficulties in recruiting and following up individuals with schizophrenia for clinical trials.
Moreover, we did not identify any study that directly compares bupropion with NRT for smoking cessation in schizophrenia. There is some evidence from both direct and indirect comparisons that the efficacy of bupropion and NRT is similar in the general population. Reference Hughes, Stead and Lancaster9 However, previous studies have suggested that smokers with normal dopaminergic function showed a better response to bupropion, whereas genotypes that are associated with impaired dopaminergic systems may have better outcomes with NRT. Reference David, Brown, Papandonatos, Kahler, Lloyd-Richardson and Munafo53,Reference Lerman, Jepson, Wileyto, Epstein, Rukstalis and Patterson54 People with schizophrenia have a dysfunctional dopaminergic system and adequately powered new RCTs that directly compare bupropion with NRT may answer the question whether these individuals respond differently to bupropion and NRT. The results of the subgroup analyses in this meta-analysis suggested that combining bupropion and NRT may increase the smoking abstinence rate compared with NRT alone. However, these analyses should be interpreted with caution because of the small number of studies involved. Nonetheless, further biochemical research regarding the mechanisms of the action of bupropion could give a better insight about the role of different neurotransmitters in nicotine addiction and withdrawal.
There are several economic analyses suggesting that bupropion is a cost-effective treatment for smoking cessation in the general public and may be more cost-effective than NRT. Reference Shearer and Shanahan55–Reference Bertram, Lim, Wallace and Vos57 None of the trials we identified included any health economic outcomes, and we are not aware of any economic analysis to investigate the cost-effectiveness of bupropion in treating nicotine dependence in people with schizophrenia. An economic analysis would allow the construction of a decision analysis algorithm, which could aid clinicians, patients and policy-makers in making evidence-based treatment decisions.
Strengths and limitations
There are a few issues to consider in this review. The number of studies was relatively small and there were some methodological weaknesses in the included trials. For example, only three trials reported using appropriate intention-to-treat analyses and we were unable to clarify methods further with the authors of other trials. Monitoring of adherence to taking medication was only reported in four trials. The results of this review also may not apply to all the people with schizophrenia, as most of the included trials explicitly excluded individuals with schizophrenia and comorbid substance misuse other than nicotine.
However, the strength of this systematic review was the use of robust methodology, including a comprehensive search strategy and an appropriate meta-analytic technique to answer an important clinical question. The results of this review should advance our current understanding of the efficacy and potential harmful effects of bupropion for smoking cessation in schizophrenia. This is the first review showing that bupropion can increase the rate of smoking abstinence in smokers with schizophrenia without jeopardising their mental state.










eLetters
No eLetters have been published for this article.