In recent years, physical health issues and specifically metabolic and cardiovascular comorbidity in different severe mental illnesses have become a major focus in both clinical care and research. Reference Bresee, Majumdar, Patten and Johnson1-Reference Stahl, Mignon and Meyer10 The association of schizophrenia with metabolic and other cardiovascular disease risk factors is a complex interplay between environmental (lifestyle, diet, substance use), genetic and illness-related factors, such as specific symptoms, as well as effects of treatment. Both older and more recent studies have confirmed the high rate of premature mortality in people with schizophrenia due to cardiovascular disease. Reference Capasso, Lineberry, Bostwick, Decker and St Sauver11-Reference Weinmann, Read and Aderhold20
The need for screening, monitoring and prevention of diabetes and other cardiovascular disease risk factors has been acknowledged in the psychiatric literature and in some of the more recent general treatment guidelines. Reference Buchanan, Kreyenbuhl, Kelly, Noel, Boggs and Fischer21-Reference Tandon, Keshavan and Nasrallah25 However, the evaluation of screening practices by clinicians has consistently shown that they are suboptimal. Reference Allochis, Cavallaro, Milano, Monteleone, Paroli and Rossi26-Reference Cohn and Sernyak37 Different national and international groups have developed guidelines relating to the monitoring and management of the increased risk for physical comorbidity in people with schizophrenia. A review in 2006 indicated substantial differences between six guidelines. Reference Cohn and Sernyak37
Guidelines on the same topic, in different domains in medicine, can differ or be in conflict with other recommendations in the same domain. Reference Ferket, Colkesen, Visser, Spronk, Kraaijenhagen and Steyerberg38 Not all guidelines have been developed with the same amount of rigour and authors' independence. Clinicians should be able to identify and have access to guidelines, which are based on the best evidence. For the individual clinician as well as services it can be difficult to identify and select a specific recommendation to use in daily practice.
The aim of this study was to perform a systematic review of the available clinical practice guidelines for the screening and monitoring of cardiometabolic risk in people with schizophrenia and related psychotic disorders. The quality of these guidelines is assessed with the Appraisal of Guidelines for Research and Evaluation (AGREE). 39-40
Method
Clinical practice guidelines for the screening and monitoring of people with schizophrenia were identified by a systematic search using PubMed, CINAHL and Embase (from 1 January 2000 until 1 April 2010) and the following search terms: Schizophrenia, Psychotic disorder, Psychosis, Mental illness, Diabetes, Cardiovascular diseases, Metabolic syndrome, Safety management and prevention, Guideline(s), Consensus development, Practice guideline(s). In the retrieved papers related articles were identified in reference lists.
Exclusion criteria were: papers only evaluating or comparing the effects of specific antipsychotic agents; general treatment guidelines for schizophrenia or psychotic disorders; guidelines specific for diabetes or cardiovascular diseases; guidelines only for children, adolescents or elderly people. All European languages were allowed if the papers met all of the following inclusion criteria: schizophrenia, cardiometabolic risk, adults and guidelines.
The evaluation and comparison of the guidelines was performed according to AGREE (2003), which is designed as a framework for the assessment of the quality of guidelines for clinical practice (www.agreecollaboration.org). 39-40
The instrument consists of 23 items grouped in six domains: scope and purpose; rigour of development; stakeholder involvement; clarity and presentation; applicability; and editorial independence. Each item is scored on a 4-point scale (strongly agree, agree, disagree and strongly disagree) with proposed anchor points to evaluate in which way the guideline fulfils the domain. The scores are standardised in a percentage score that enables comparison between guidelines (obtained score–minimum possible score)/(maximum possible score–minimum possible score). The final component of the AGREE instrument involves a recommendation regarding the use of the guidelines in practice as ‘recommended’, ‘recommended (with provisos or exceptions)’, ‘would not recommend or unsure’, depending on the number of items and domains if the score was >60%, 30-60% and <30%, respectively. Three raters (D.V., K.S. and M.D.H.) independently scored the identified guidelines (M.D.H. acknowledges a potential conflict of interest because he co-authored two of the assessed guidelines). A mean score was calculated for each item from which the percentage score was derived according to the AGREE manual. In addition, each guideline was independently evaluated regarding the specific content and scope of what should be monitored by whom. Process indicators were predefined and scored on a standardised scoring sheet (online supplement 1). Intraclass correlation coefficients (ICC) with a 95% confidence interval were calculated as an overall indicator of agreement among the raters for each of the 23 items of the AGREE instrument. Reference Landis and Koch41
Results
The initial search with all search terms yielded 4608 hits (Fig. 1). That number was reduced to 18 when the specific inclusion criteria were applied. A total of 54 guidelines were found of which 35 were excluded: 23 duplicate papers (either by the same or different authors with similar guideline content in different journals); Reference Cohn and Sernyak37,42-Reference Sernyak63 4 general schizophrenia treatment guidelines; Reference Kreyenbuhl, Buchanan, Dickerson and Dixon23,24,64,Reference Mcgorry, Killackey, Lambert and Lambert65 3 guidelines only for children or adolescents; Reference Correll, Penzner, Parikh, Mughal, Javed and Carbon66-Reference Dobbelaere and De Hert68 2 for people with bipolar disorder; Reference Bobes, Sáiz, Montes, Mostaza and Rico-Villademoros69-Reference Ng, Mammen, Wilting, Sachs, Ferrier and Cassidy70 and 3 diabetes guidelines. 71-Reference Woo, Harris and Houlden73 One guideline for metabolic screening in people with schizophrenia was excluded because it was only available in Japanese and we were not able to get it translated. Reference Murasaki, Koyama, Atsumi and Kadowaki74
A total of 18 unique guidelines were identified for AGREE evaluation either from the USA (2), Australia (2), Brazil (1), Canada (1) or Europe (12), and all were published between 2004 and 2010 (online Table DS1). 75-Reference Usher, Foster and Park92 All papers covered diabetes and cardiovascular disease risk in individuals treated with antipsychotic agents, whereas some had a broader scope also including other physical health domains and other side-effects. Reference Cahn, Ramlal, Bruggeman, de Haan, Scheeepers and van Soest78,Reference Lefebvre, Chereau, Schmitt and Llorca85,Reference Marder, Essock, Miller, Buchanan, Casey and Davis86,Reference Saiz, Bobes, Vallejo, Giner and Garcia-Portilla89,Reference Saravane, Feve, Frances, Corruble, Lancon and Chanson91
Interrater reliability
The overall ICC value among the observers was 0.89 (95% CI 0.88-0.91, P<0.0001). None of the scores on individual items for all assessed guidelines differed more than one point on the AGREE scale.
Evaluation of the quality of the guidelines
There was wide variation in standardised scores of the different domains (online Table DS1). Apart from stakeholder involvement, all of the domains had a difference of at least 45% between the highest and lowest scoring guideline. Only two domains had a mean score above 50% (scope and purpose, clarity and presentation).
The highest mean domain score, derived from pooled scores, was for scope and purpose (56.4%) with five guidelines scoring below 40% and six having a score of 70% or above. The highest score, 81.5%, was achieved by three guidelines. 75,Reference Barnett, Mackin, Chaudhry, Farooqi, Gadsby and Heald77,Reference De Hert, Dekker, Wood, Kahl, Holt and Möller79
Clarity and presentation was satisfactory in most guidelines (50.6%), only four had a score of 31% or lower. All the guidelines with a score above 50% presented a clear table or figure, summarising the proposed screening content and time intervals.
Regarding the domain rigour of development all except one guideline had a score below 50%. Reference Saiz, Bobes, Vallejo, Giner and Garcia-Portilla89 Although some guidelines presented data from a systematic review of the literature, the search strategy for literature selection was missing in all but one guideline. Reference Saiz, Bobes, Vallejo, Giner and Garcia-Portilla89 Only two guidelines presented levels/quality of the evidence Reference Lambert and Chapman84,Reference Marder, Essock, Miller, Buchanan, Casey and Davis86 and one presented meta-analytic data. Reference Saiz, Bobes, Vallejo, Giner and Garcia-Portilla89 More than half (61%) of the guidelines were developed with a consensus model (online Table DS2). Within this domain the criterion about the updating of the recommendation was not fulfilled by any of the guidelines. The older UK guideline has a low score on this item, but the paper was published in a themed issue of the journal, with different papers presenting a systematic review of the literature in that same issue. Reference Dinan, Holt, Kohen, Thakore, Haddad and Baker81
Scores in the application domain were satisfactory in five guidelines. The guidelines with a low score on this domain failed to discuss the organisational aspects of introducing screening and monitoring. Health economic aspects were mentioned in some guidelines but the additional cost of screening and monitoring was explicitly available in only one. Reference Saravane, Feve, Frances, Corruble, Lancon and Chanson91
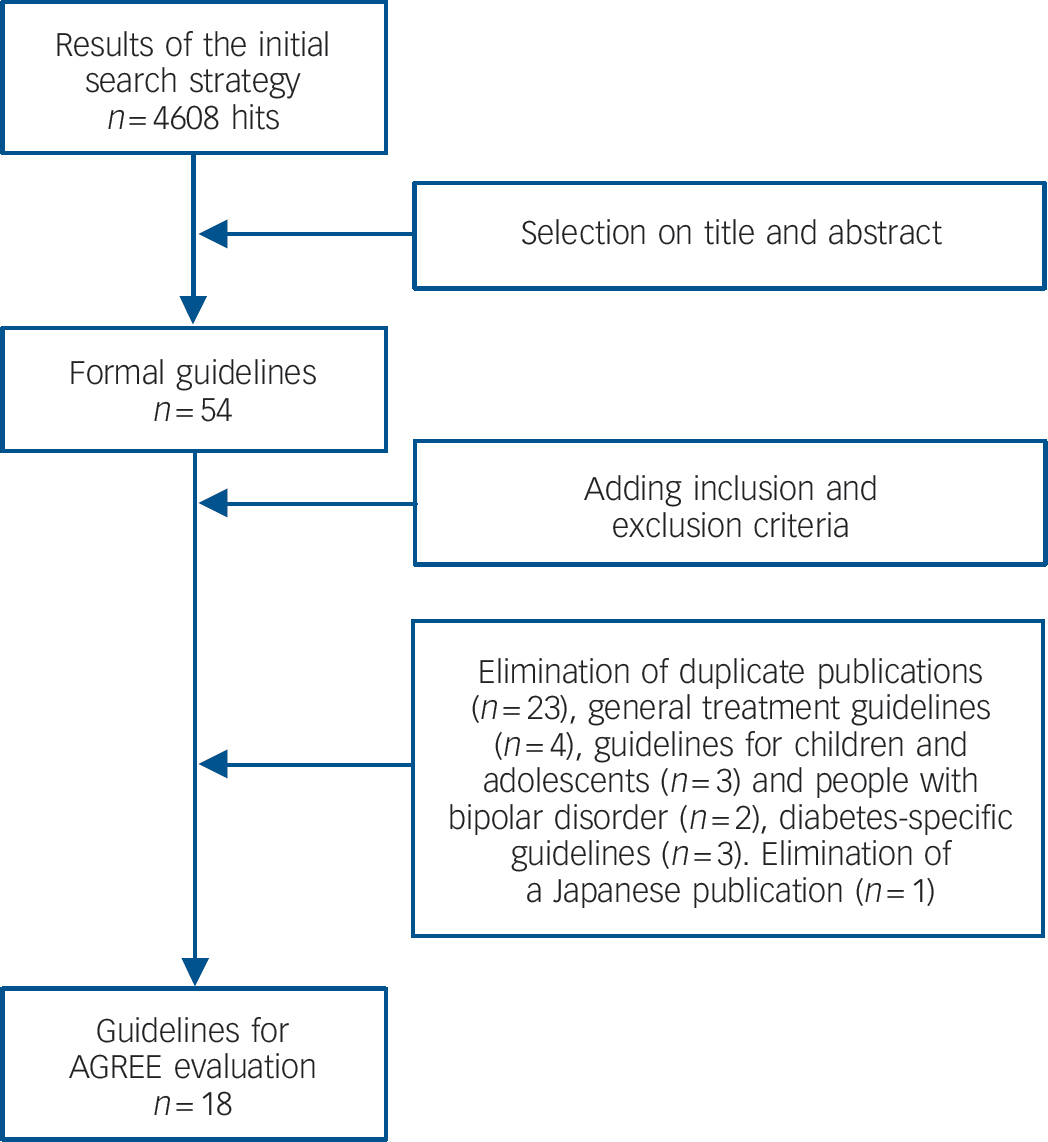
Fig. 1 Results of the systematic literature search.
Editorial independence was the only domain with a range of scores from 0 to 100%. Eight guidelines had industry involvement and in only seven there was a declaration of conflicts of interest. Some publications were not industry sponsored but lacked a conflict of interest section resulting in a low AGREE score on this item (online Table DS2).
The lowest mean domain score was for stakeholder involvement (30.6%), with only six guidelines scoring above 30%. Only in two guidelines had patients been involved in the guidelines' development. The proposed guidelines were never tested on the intended users. Except for three, Reference Amati, Biondi, Bogetto, Casacchia, Castrogiovanni and Giorgino76,Reference Lefebvre, Chereau, Schmitt and Llorca85,Reference Usher, Foster and Park92 all the guidelines were developed by a multidisciplinary group. Official medical societies were involved in the oldest 75 and in six of the seven most recent guidelines (online Table DS2). Reference Cahn, Ramlal, Bruggeman, de Haan, Scheeepers and van Soest78,Reference De Hert, Dekker, Wood, Kahl, Holt and Möller79,Reference Elkis, Gama, Suplicy, Tambascia, Bressan and Lyra82,Reference Gothefors, Adolfsson, Attvall, Erlinge, Jarbin and Lindström83,Reference Saiz, Bobes, Vallejo, Giner and Garcia-Portilla89,Reference Salokangas, Hirvonen, Honkonen, Jyväsjärvi, Koponen and Laukkale90
Comparing the guidelines across the overall scores in the different domains, four European Reference Barnett, Mackin, Chaudhry, Farooqi, Gadsby and Heald77,Reference De Hert, Dekker, Wood, Kahl, Holt and Möller79,Reference Gothefors, Adolfsson, Attvall, Erlinge, Jarbin and Lindström83,Reference Saiz, Bobes, Vallejo, Giner and Garcia-Portilla89 and one US Reference Marder, Essock, Miller, Buchanan, Casey and Davis86 guideline could be recommended, whereas four guidelines failed in nearly all domains. Reference Amati, Biondi, Bogetto, Casacchia, Castrogiovanni and Giorgino76,Reference Lefebvre, Chereau, Schmitt and Llorca85,Reference Melkersson, Dahl and Hulting87,Reference Usher, Foster and Park92
Guideline development process
Online Table DS2 presents details about the guideline development process. Most were based on a selective or systematic literature review. A majority was based on a consensus model involving different medical disciplines. People with schizophrenia were the target for the proposed recommendations in all guidelines, although some broadened the scope to people with other severe mental illnesses being treated with antipsychotic medication. Four guidelines specifically mentioned paediatric patients as a vulnerable group. 75,Reference Cahn, Ramlal, Bruggeman, de Haan, Scheeepers and van Soest78,Reference De Hert, Dekker, Wood, Kahl, Holt and Möller79,Reference Gothefors, Adolfsson, Attvall, Erlinge, Jarbin and Lindström83
Recommended assessment of risk factors for diabetes and cardiovascular disease
All but one Reference Salokangas, Hirvonen, Honkonen, Jyväsjärvi, Koponen and Laukkale90 guideline recommended assessment of family history, and three Reference Lefebvre, Chereau, Schmitt and Llorca85,Reference Melkersson, Dahl and Hulting87,Reference Salokangas, Hirvonen, Honkonen, Jyväsjärvi, Koponen and Laukkale90 failed to include personal history (online Table DS3). Only 56% of the guidelines proposed a general physical examination of the patient. Assessment of other known risk factors for diabetes and cardiovascular disease was incomplete in most of the guidelines; 44% even failed to mention smoking. Three included a comprehensive risk assessment. Reference De Hert, Dekker, Wood, Kahl, Holt and Möller79,Reference De Nayer, De Hert, Scheen, Van Gaal and Peuskens80,Reference Saravane, Feve, Frances, Corruble, Lancon and Chanson91
Most of the guidelines stated that the frequency of monitoring is dependent on the presence of risk factors (including being overweight and obesity) and on the time since starting the antipsychotic medication (more frequent monitoring when close to starting medication) (online Table DS4). A minority of guidelines (28%) suggested that the frequency of monitoring is dependent on specific antipsychotic agents. In most cases, they stipulated that it is the responsibility of the psychiatrist/prescriber to ensure that the screening and monitoring is being conducted. Six guidelines explicitly also involved a general practitioner (GP) in this responsibility and promoted models of shared care. Reference De Hert, Dekker, Wood, Kahl, Holt and Möller79-Reference Dinan, Holt, Kohen, Thakore, Haddad and Baker81,Reference Gothefors, Adolfsson, Attvall, Erlinge, Jarbin and Lindström83-Reference Saravane, Feve, Frances, Corruble, Lancon and Chanson91
Comparison of recommendations in the guideline
Overall, the recommendations across guidelines were more similar than dissimilar regarding variables that should be assessed both at baseline and over time (online Table DS5). Apart from weight and body mass index, which were present in most guidelines (89%), a majority of guidelines also included measurements of waist circumference (83%). All guidelines also included the assessment of fasting glucose (online Table DS5), and the majority (89%) also included the assessment of fasting lipids (online Table DS5). The major differences between guidelines were in the timing and the interval between assessments. The more recent guidelines were more detailed and included additional evaluations about personal and family history and cardiovascular risk factors. Six guidelines (33%) failed to mention blood pressure monitoring. A substantial number of them also failed to mention specific timing for screening or to mention cut-off or target values for the assessed variables (most frequently for the lipid measurements). For glucose values the reference values presented were either based on the American Diabetes Association or World Health Organization thresholds at the time of publication. For lipid values, the cut-offs of the Adult Treatment Panel were used most frequently. In 60% of the guidelines, the reference values were based on the recommendations of official societies (either cardiology or diabetology).
For glucose abnormalities, four guidelines mentioned the possibility of assessing non-fasting glucose (although they, at the same time, recommended assessing fasting lipid profile) and only two recommended glycosylated haemoglobin (HbA1c). Oral glucose tolerance tests were recommended in six; in case of impaired fasting glucose values. Reference Barnett, Mackin, Chaudhry, Farooqi, Gadsby and Heald77,Reference De Nayer, De Hert, Scheen, Van Gaal and Peuskens80,Reference Elkis, Gama, Suplicy, Tambascia, Bressan and Lyra82-Reference Lambert and Chapman84,Reference Poulin, Cortese, Williams, Wine and Mcintyre88 Monitoring for signs and symptoms of diabetes was recommended by different guidelines (67%), whereas only a few (33%) explicitly mentioned assessing diabetic ketoacidosis (online Table DS6). The concept of the metabolic syndrome was formally included in only four.
Guidelines with a more global physical health scope recommended additional laboratory testing, ranging from prolactin levels to viral serology and different additional somatic investigations (online Table DS6). In total, 50% proposed ECG monitoring, at least when using drugs with a potential for QTc prolongation.
Therapeutic recommendations varied substantially across the different domains assessed (online Table DS7). Most guidelines recommended education of patients (83%), although only 50% involved the family (online Table DS7). A small majority (56%) recommended switching of antipsychotic agents in the case of significant increases in risk factors. A minority (33%) considered the initial choice of a specific antipsychotic to be important. Advice on diet and exercise was present in most guidelines, but recommendations were rarely specific enough to guide clinical practice. Only 50% of them recommended promoting smoking cessation.
In most of the guidelines, treatment for diabetes and lipid abnormalities was addressed, recommending that individuals with significant abnormalities be referred to a GP or a medical specialist for evaluation and/or treatment. Only six (33%) considered treatment for hypertension. Educational interventions for mental health workers, GPs or medical specialists were rarely proposed (online Table DS7).
Discussion
This is the first study that systematically evaluates the content and quality of practice guidelines for the screening and monitoring of diabetes and cardiovascular risk in people with schizophrenia. For the qualitative and quantitative assessment of these guidelines, we used AGREE, a widely used and accepted tool for the quality assessment of such material. 39,40 The assessed guidelines differed significantly regarding the different AGREE domains. The highest scores were obtained for scope and purpose and clarity of presentation. The lowest scores were found for the domains stakeholder involvement and rigour of development. There was less difference between the basic sets of variables that should be assessed in patients, but substantial differences were apparent in the level of detail and timing of the recommended monitoring and proposed therapeutic strategies.
Clinical recommendations
After a baseline assessment, 10 of the 18 guidelines recommended monitoring after the first 3-4 months of treatment, but 4 recommended monitoring after 4-6 weeks and 1 required only monitoring at 6 months. The following measurements were recommended, in order of frequency: fasting glucose, body mass index, fasting triglycerides, fasting cholesterol, waist, high density lipoprotein/low density lipoprotein, blood pressure and symptoms of diabetes. In terms of interventions, most guidelines recommended advice on physical activity, advice on diet, psychoeducation of the patient, treatment of lipid abnormalities, treatment of diabetes, referral for advice and treatment, psychoeducation of the family and smoking cessation advice. Of the screening tests, fasting glucose, fasting triglycerides and fasting cholesterol may be less easily integrated into routine care because of the need to organise fasting blood tests. Thus compliance with such tests is often less than 20%. Reference Buckley, Miller and Singer28,Reference Haupt, Rosenblatt, Kim, Baker, Whitehead and Newcomer29,Reference Morrato, Newcomer, Allen and Valuck31 Alternatives such as nonfasting HbA1c are promising but require further validation in psychiatric settings. Reference Henderson, Cagliero, Copeland, Louie, Borba and Fan93-95
Implementation of guidelines
Clinical practice guidelines are considered a good option for translating research into clinical practice. They are defined as ‘systematically developed statements to assist practitioner and patient decisions about appropriate healthcare for specific clinical circumstances'’. 96 Their potential to improve patient care and outcomes depends largely on the quality and independence of the guideline. Reference Woolf, Grol, Hutchinson, Eccles and Grimshaw97 Recommendations may be biased because of non-systematic selection, inadequate interpretation or lack of scientific evidence. The content may initially be decided through consensus, whereas scientific evidence to support the consensus is added afterwards. The influence of the context within which the guidelines are produced (for example by medical societies or with support of pharmaceutical companies) has also been mentioned in relation to the variation across guidelines. Reference Woolf, Grol, Hutchinson, Eccles and Grimshaw97-Reference Shekelle, Woolf, Eccles and Grimshaw99
Quality evaluations have recently been performed for other diseases in relation to metabolic and cardiovascular risk monitoring. Reference Delgado-Noguera, Tort, Bonfill, Gich and Alonso-Coello100-Reference Graham, Atar, Borch-Hohnsen, Boysen, Burell and Cifkova102 Similar to our findings, for diabetes and cardiovascular disease the rigour of development and other quality indications, such as stakeholder involvement and editorial independence, were not ideal in a number of these guidelines. This was the case, despite medical societies developing stringent methodologies for these diseases according to internal guidelines/procedures. Reference MacDermid, Brooks, Solway, Switzer-McIntyre, Brosseau and Graham103 Moreover, editorial independence was also often a problematic area, and frequently guidelines were not based on high-quality evidence. Reference Ferket, Colkesen, Visser, Spronk, Kraaijenhagen and Steyerberg38,Reference McAlister, van Diepen, Padwal, Johnson and Majumdar98-Reference Vlayen, Aertgeerts, Hannes, Sermeus and Ramaekers104
Limitations
A limitation of the AGREE methodology is the degree of subjective judgement. Reference Delgado-Noguera, Tort, Bonfill, Gich and Alonso-Coello100,Reference Stone, Wilkinson, Charpentier, Clochard, Grassi and Lindblad101 This can be partly overcome through the evaluation by different independent reviewers, as was done in this study, and with high interrater reliability. We did not contact
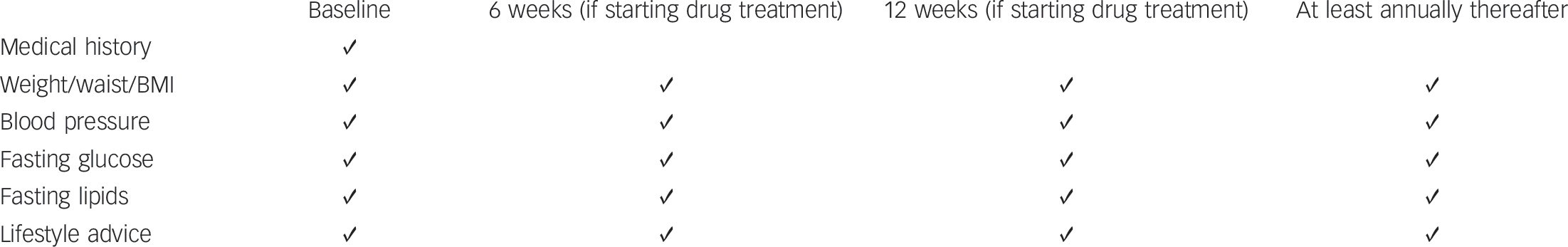
Fig. 2 Monitoring protocol for managing individuals with normal baseline values at start of an episode of care.
The 6-week assessment has only been endorsed in some European guidelines and the advantages of this additional, early assessment time point still have to be demonstrated. Body mass index (BMI): during initial phases of treatment, it is important to measure weight weekly to identify individuals who may be gaining weight rapidly.
the original authors for additional information. This could have been relevant for those guidelines not having industry sponsoring, but failing to report conflicts of interest (in the appraisal, such guidelines received a score of ‘totally disagree’ on item 22). Furthermore, the AGREE evaluation does not evaluate the impact of the guidelines on patient outcome. Reference Delgado-Noguera, Tort, Bonfill, Gich and Alonso-Coello100
Authors of a recent joint paper on the Schizophrenia Patient Outcomes Research Team (PORT) guidelines acknowledged the difficulty of getting published guidelines to change clinical practice. Reference Pincus105 This is supported by a number of studies. So far all studies that assessed the impact of the American Diabetes Association/American Psychiatric Association (ADA/APA) 2004 guidelines 75 in the USA, which went hand in hand with extensive educational efforts, suggest that the impact on real-life screening and monitoring rates of people receiving antipsychotics in different population samples in the USA is minimal to poor. Reference Buckley, Miller and Singer28-Reference Morrato, Nicol, Maahs, Druss, Hartung and Valuck34 Similar results have also emerged in the UK. Reference Barnes, Paton, Cavanagh, Hancock and Taylor27 Of note, although often calling for research, none of the guidelines explicitly mentioned an update procedure. The only guideline that has undergone an extensive review and update process is the ADA/APA consensus document. 75 A new version of this guideline is currently being finalised.
General conclusions
Overall, we conclude that several adequate guidelines for screening and monitoring are available. The published guidelines all focus on the same evidence base but differ mainly regarding the timing of monitoring and the scope of physical health domains that are to be monitored. Comparing the guidelines across the overall scores in the different domains of the AGREE assessment and taking into account the level of detail and comprehensiveness of the monitoring, four European guidelines can be recommended for clinical use in daily practice. Reference Barnett, Mackin, Chaudhry, Farooqi, Gadsby and Heald77,Reference De Hert, Dekker, Wood, Kahl, Holt and Möller79,Reference Gothefors, Adolfsson, Attvall, Erlinge, Jarbin and Lindström83,Reference Saiz, Bobes, Vallejo, Giner and Garcia-Portilla89 However, none of the guidelines had a proposed schedule for an update and they all require more rigorous implementation strategies, together with studies into the impact on actual screening rates; long-term patient outcomes should also be put in place.
Based on this review of the guidelines, a monitoring protocol for managing cardiovascular disease risk in patients in clinical practice is proposed in Fig. 2. All individuals with schizophrenia should be under active care (regardless of treatment condition) and be screened at least annually if they have normal baseline values. Those who already present with cardiovascular risk factors should be monitored more frequently. At the start of a new treatment, assessments should be repeated 6 and 12 weeks after initiation of the new antipsychotic drug treatment (the 6-week assessment has only been endorsed in some European guidelines and the advantages of this additional, early assessment time point still have to be demonstrated). These recommendations are in general agreement with the National Institute for Health and Clinical Excellence guidelines, 24 which stress the need to prevent cardiovascular disease and to undertake metabolic risk assessment, but do not provide clear guidance on which evaluations need to be done when, and with the Canadian Diabetes Association clinical practice guidelines, which considers schizophrenia as a risk factor for diabetes. 71 Based on a systematic review of the metabolic effects of antipsychotics in children and adolescents, similar guidelines for cardiometabolic screening have been proposed recently. Reference De Hert, Dobbelaere, Sheridan, Cohen and Correll106 Based on recent data in drug-naive individuals Reference Alvarez-Jimenez, Gonzalez-Blanch, Crespo-Facorro, Hetrick, Rodriguez-Sanchez and Perez-Iglesias107-Reference Tarricone, Gozzi, Serretti, Grieco and Berardi109 and recent general treatment guidelines, Reference Buchanan, Kreyenbuhl, Kelly, Noel, Boggs and Fischer21,Reference Kreyenbuhl, Buchanan, Dickerson and Dixon23,Reference De Hert, Dobbelaere, Sheridan, Cohen and Correll106 antipsychotic medications with a high liability to induce metabolic changes are not recommended to be used as first-line agents in first-epi-sode/never exposed individuals.
As in other healthcare domains, improvement is needed in the quality of the guidelines for screening and monitoring cardiometabolic risks in people with schizophrenia. In order for this shift to happen, guidelines should adhere more closely to the methodology proposed in the AGREE instrument. Finally, due to the increasing burden of obesity among individuals with schizophrenia and the potential for long-term cardiometabolic comorbidities, clinicians need to have access to key recommendations from the best available guidelines, be critical of how these are developed and consider their appropriateness for use in their own clinical practice.







eLetters
No eLetters have been published for this article.