Analysis of cortical thinning from high-resolution magnetic resonance imaging (MRI) scans has contributed to a better understanding of cortical morphological effects of schizophrenia on the brain. Initial studies of people with mostly chronic schizophrenia demonstrated inferior and medial frontal as well as inferior and medial temporal cortical thinning Reference Kuperberg, Broome, McGuire, David, Eddy and Ozawa1 and several subsequent studies have confirmed cortical thinning in frontal and temporal areas to varying degrees; this has included first-episode Reference Crespo-Facorro, Roiz-Santianez, Perez-Iglesias, Rodriguez-Sanchez, Mata and Tordesillas-Gutierrez2–Reference Schultz, Koch, Wagner, Roebel, Schachtzabel and Gaser5 as well as chronic patient groups. Reference Rimol, Nesvag, Hagler, Bergmann, Fennema-Notestine and Hartberg6,Reference Palaniyappan and Liddle7 Several subsequent imaging studies on cortical thickness have suggested that both genetic factors and those related to progressive components in the disorder might have an impact on cortical thinning. Studies in siblings or relatives of patients have produced mixed results, with some suggesting cortical thinning in temporal and/or frontal areas Reference Sprooten, Papmeyer, Smyth, Vincenz, Honold and Conlon4,Reference Oertel-Knochel, Knochel, Rotarska-Jagiela, Reinke, Prvulovic and Haenschel8–Reference Karnik-Henry, Wang, Barch, Harms, Campanella and Csernansky10 and others not supporting this finding. Reference Boos, Cahn, van Haren, Derks, Brouwer and Schnack11,Reference Yang, Nuechterlein, Phillips, Hamilton, Subotnik and Asarnow12 However, several studies have now reported associations of cortical thinning with specific schizophrenia risk genes, Reference Tandon, Gaebel, Barch, Bustillo, Gur and Heckers13–Reference Schultz, Nenadic, Koch, Wagner, Roebel and Schachtzabel18 although effect sizes and their ultimate impact on total variability of cortical thickness remains unclear. Studies using other methods for assessment of cortical brain structure, such as voxel-based morphometry (VBM), have recently demonstrated that structural deficits might vary as a function of distinct subtypes of schizophrenia. Two large studies have demonstrated that dividing schizophrenia cohorts into three groups, such that groupings are based on dominant symptoms, reveals patterns of substantial variability in frontal and temporal subregions as well as overlap across groups. Reference Koutsouleris, Gaser, Jager, Bottlender, Frodl and Holzinger19,Reference Nenadic, Sauer and Gaser20 We have also recently replicated this effect of subgroup-related variability in a measure of cortical complexity. Reference Nenadic, Yotter, Sauer and Gaser21
Regarding, cortical thinning, a recent study has shown effects related to the deficit syndrome of schizophrenia, Reference Takayanagi, Wentz, Takayanagi, Schretlen, Ceyhan and Wang22 but it remains unclear whether these effects are related to patterns of symptomatology, and thus possibly to a distinct phenotype, or whether it is rather related to intermediate phenotype markers of the disorder. In the present study, we tested the hypothesis that subgroups of patients with schizophrenia, delineated by their symptom profiles, would show different extents of cortical thickness changes, especially in prefrontal and temporal cortical areas. Identifying areas of overlap and divergence would highlight the role of cortical thickness as a putative intermediate phenotype. Reference Nenadic, Gaser and Sauer23 In order to compare cortical thickness with other markers, we used the same approach for subdividing patients with schizophrenia as used in previous VBM studies. Reference Koutsouleris, Gaser, Jager, Bottlender, Frodl and Holzinger19,Reference Nenadic, Sauer and Gaser20
Method
Participants
We studied a cohort of 87 patients with DSM-IV 24 schizophrenia and 108 healthy controls, all of whom had provided written informed consent to a protocol approved by the Ethics Committee of the Medical School of Friedrich-Schiller-University, Jena. All participants were adults and were right-handed. Reference Oldfield25 The sample was drawn from a slightly larger cohort of 99 patients and 113 healthy controls, for which VBM analyses were presented previously, Reference Nenadic, Sauer and Gaser20 and identical to a sample in a recent analysis of a novel cortical complexity measure. Reference Nenadic, Yotter, Sauer and Gaser21 Although all of the initial patients and controls passed the VBM quality control protocol, 12 participants had to be excluded based on our quality control protocol for cortical surface-based measures.
Patients were recruited from in-patient and out-patient facilities of the Jena University Hospital’s Department of Psychiatry, and underwent diagnostic assessment by a psychiatrist, who also rated the severity of positive and negative symptoms on the Scale for Assessment of Positive Symptoms (SAPS) Reference Andreasen26 and Scale for Assessment of Negative Symptoms (SANS) Reference Andreasen26 respectively at the time of scanning (same week). All patients were on stable antipsychotic medication. Healthy controls had no concurrent or previous psychiatric disorder, nor major medical or neurological conditions.
Both the SANS and SAPS scores (using single items) were used to derive a delineation of three subgroups based on factor analysis with Promax rotation. Delination was based on the larger initial sample of 99 patients and 113 healthy controls. The three-subgroup delineation was validated in previous studies by Liddle et al. Reference Liddle, Friston, Frith, Hirsch, Jones and Frackowiak27,Reference Liddle and Morris28 It also is temporally stable, found even in older patients with chronic schizophrenia. Reference Sauer, Hornstein, Richter, Mortimer and Hirsch29
The subgroups consisted of: (a) 31 participants with predominantly negative symptoms (negative symptoms subgroup; 16 female, 15 male; mean age 35.54 years, s.d. = 11.17); (b) 23 participants with predominantly disorganised symptoms (disorganised symptoms subgroup; 9 female, 14 male; mean age 35.39 years, s.d. = 11.13); and (c) 33 participants with predominantly paranoid symptoms (paranoid symptoms subgroup; 14 female, 19 male; mean age 35.54 years, s.d. = 11.18). These subgroups did not differ significantly from the control group (40 females, 68 males; mean age 32.16 years, s.d. = 9.99) in age or gender (t-tests for age: control group v. negative symptoms subgroup, P = 0.135; control group v. disorganised symptoms subgroup, P = 0.209; control group v. paranoid symptoms subgroup, P = 0.126; χ2 for gender: control group v. negative symptoms subgroup, P = 0.145; control group v. disorganised symptoms subgroup, P = 0.852; control group v. paranoid symptoms subgroup, P = 0.578).
Psychopathology scores for the three subgroups, as reported previously, Reference Nenadic, Yotter, Sauer and Gaser21 were as follows. Negative symptoms subgroup: SAPS mean 11.5 (s.d. = 11.7), SANS mean 44.6 (s.d. = 14.1); disorganised symptoms subgroup: SAPS mean 24.3 (s.d. = 14.0), SANS mean 35.7 (s.d. = 16.5); paranoid symptoms subgroup: SAPS mean 22.6 (s.d. = 18.9), SANS mean 27.8 (s.d. = 17.8). Also, there was no difference between subgroups for disease duration (negative symptoms subgroup: mean 8.07 years, s.d. = 8.11; disorganised symptoms subgroup: mean 10.14 years, s.d. = 7.68; paranoid symptoms subgroup: mean 7.29 years, s.d. = 7.44; ANOVA not significant) or age at onset (negative symptoms subgroup: mean 28.25 years, s.d. = 9.27; disorganised symptoms subgroup: mean 26.13 years, s.d. = 11.6; paranoid symptoms subgroup: mean 30.23 years, s.d. = 9.82; ANOVA not significant).
Image acquisition and data analysis
We analysed high-resolution T 1-weighted MRI scans, which were acquired for each participant on a 1.5 Tesla Phillips Gyroscan ASCII scanner (repetition time (TR) = 13 ms, echo time (TE) = 5 ms, 256 slices in sagittal orientation, field-of-view 256 mm; isotropic voxel resolution: 1×1×1 mm3). Computation of cortical thickness was done using FreeSurfer software (v4.3 for Mac OS X) as described previously. Reference Nenadic, Yotter, Sauer and Gaser21 In brief, we applied intensity normalisation, skull stripping and alignment for head position along the commissural axis, as well as labelling of cortical and subcortical structures. Reference Dale, Fischl and Sereno30–Reference Fischl, Sereno, Tootell and Dale32 Quality control included visual inspection for gross imaging artefacts, as well as for poor quality of the extraction of the cortical surface. All scans included in this study used fully-automated extraction of the cortical surface with excellent results.
For statistical analysis, we tested differences in cortical thickness at three levels using separate general linear models (all implemented in a Matlab routine (Matlab 2010b), based on Statistical Parametric Mapping (SPM8) statistical routines): global thickness (across each hemisphere), regional thickness (across regions of interests (ROIs), defined by the Desikan atlas), and vertex-wise. For regional analysis, which constituted our main analysis, we applied a P<0.05 threshold for significance with false discovery rate (FDR) to adjust for multiple comparisons, and also included gender as a nuisance variable, since this variable might induce moderate effects even in the absence of group-level differences in sample composition. Differences were first tested between the whole patient group and the control group, and then between each of the three subgroups (negative, disorganised and paranoid symptoms) and the control group (P<0.05, FDR-adjusted).
Results
Patients with schizophrenia showed cortical thinning on global (hemispheric), regional and vertex-wise levels. Cortical thinning was evident across both left and right hemispheres, and was highly significant (Table 1). The effect was most pronounced for the negative symptoms subgroup.
Table 1 Global (hemisphere) analysis of cortical thickness for control and schizophrenia groups
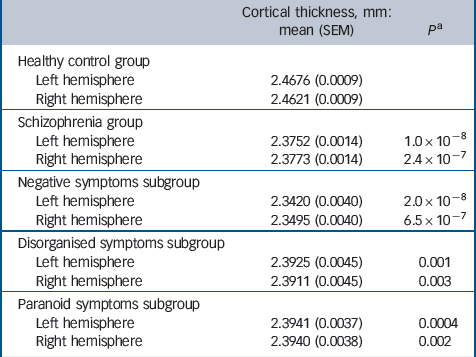
| Cortical thickness,
mm: mean (SEM) |
P Footnote a | |
|---|---|---|
| Healthy control group | ||
| Left hemisphere | 2.4676 (0.0009) | |
| Right hemisphere | 2.4621 (0.0009) | |
| Schizophrenia group | ||
| Left hemisphere | 2.3752 (0.0014) | 1.0 × 10–8 |
| Right hemisphere | 2.3773 (0.0014) | 2.4 × 10–7 |
| Negative symptoms subgroup | ||
| Left hemisphere | 2.3420 (0.0040) | 2.0 × 10–8 |
| Right hemisphere | 2.3495 (0.0040) | 6.5 × 10–7 |
| Disorganised symptoms subgroup | ||
| Left hemisphere | 2.3925 (0.0045) | 0.001 |
| Right hemisphere | 2.3911 (0.0045) | 0.003 |
| Paranoid symptoms subgroup | ||
| Left hemisphere | 2.3941 (0.0037) | 0.0004 |
| Right hemisphere | 2.3940 (0.0038) | 0.002 |
SEM, standard error of the mean.
a. Comparison with the control group.
Our main analysis of regional effects across the three subgroups (Table 2) showed that the negative symptoms subgroup showed significant cortical thinning (P<0.05, FDR-adjusted) in prefrontal and temporal areas, as well as almost all other parietal and occipital cortical regions. The paranoid symptoms subgroup also showed significant thinning across several prefrontal and temporal areas, with sparing of the temporopolar, frontopolar and bilateral cuneus, whereas effects in inferior temporal and superior parietal areas varied across hemispheres. The disorganised symptoms subgroup showed the least cortical thinning, but still revealed several significantly thinner regions, including the superior and middle prefrontal, as well as temporal cortices.
Table 2 Regional (ROI) analysis of cortical thickness in the control group, the schizophrenia group, and the negative, disorganised and paranoid symptoms subgroupsFootnote a
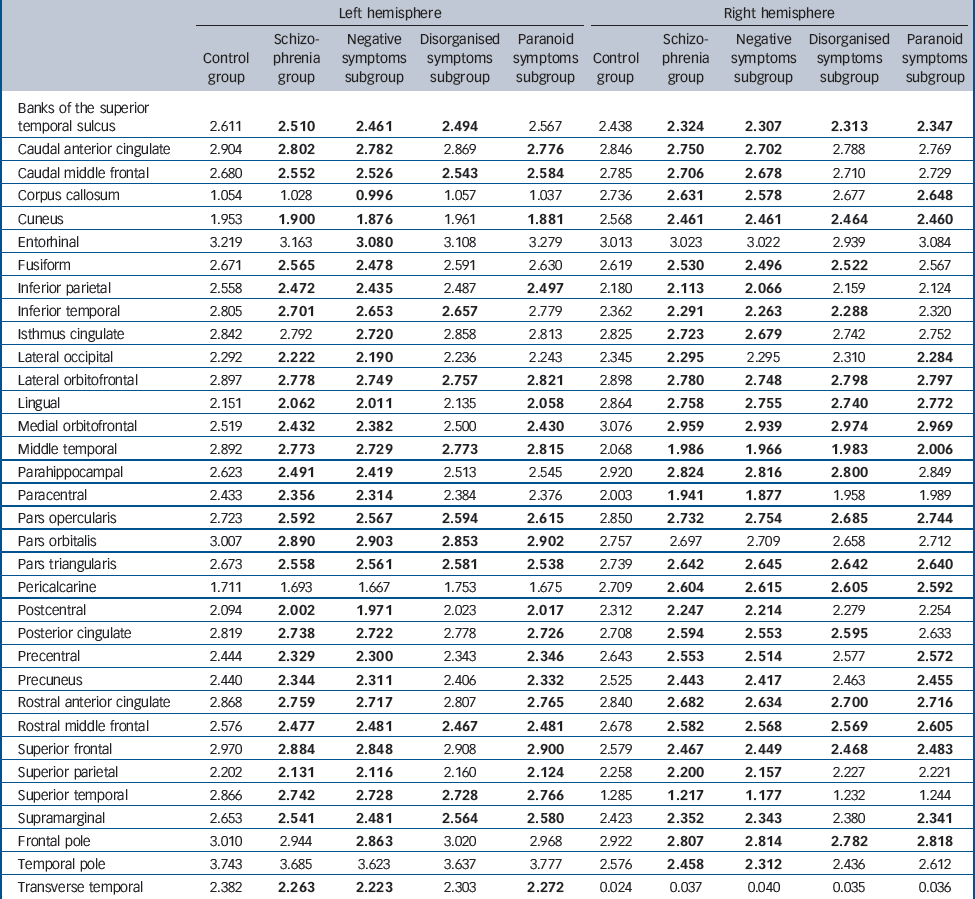
| Left hemisphere | Right hemisphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control group |
Schizo- phrenia group |
Negative symptoms subgroup |
Disorganised symptoms subgroup |
Paranoid symptoms subgroup |
Control group |
Schizo- phrenia group |
Negative symptoms subgroup |
Disorganised symptoms subgroup |
Paranoid symptoms subgroup |
|
| Banks of the superior temporal sulcus |
2.611 | 2.510 | 2.461 | 2.494 | 2.567 | 2.438 | 2.324 | 2.307 | 2.313 | 2.347 |
| Caudal anterior cingulate | 2.904 | 2.802 | 2.782 | 2.869 | 2.776 | 2.846 | 2.750 | 2.702 | 2.788 | 2.769 |
| Caudal middle frontal | 2.680 | 2.552 | 2.526 | 2.543 | 2.584 | 2.785 | 2.706 | 2.678 | 2.710 | 2.729 |
| Corpus callosum | 1.054 | 1.028 | 0.996 | 1.057 | 1.037 | 2.736 | 2.631 | 2.578 | 2.677 | 2.648 |
| Cuneus | 1.953 | 1.900 | 1.876 | 1.961 | 1.881 | 2.568 | 2.461 | 2.461 | 2.464 | 2.460 |
| Entorhinal | 3.219 | 3.163 | 3.080 | 3.108 | 3.279 | 3.013 | 3.023 | 3.022 | 2.939 | 3.084 |
| Fusiform | 2.671 | 2.565 | 2.478 | 2.591 | 2.630 | 2.619 | 2.530 | 2.496 | 2.522 | 2.567 |
| Inferior parietal | 2.558 | 2.472 | 2.435 | 2.487 | 2.497 | 2.180 | 2.113 | 2.066 | 2.159 | 2.124 |
| Inferior temporal | 2.805 | 2.701 | 2.653 | 2.657 | 2.779 | 2.362 | 2.291 | 2.263 | 2.288 | 2.320 |
| Isthmus cingulate | 2.842 | 2.792 | 2.720 | 2.858 | 2.813 | 2.825 | 2.723 | 2.679 | 2.742 | 2.752 |
| Lateral occipital | 2.292 | 2.222 | 2.190 | 2.236 | 2.243 | 2.345 | 2.295 | 2.295 | 2.310 | 2.284 |
| Lateral orbitofrontal | 2.897 | 2.778 | 2.749 | 2.757 | 2.821 | 2.898 | 2.780 | 2.748 | 2.798 | 2.797 |
| Lingual | 2.151 | 2.062 | 2.011 | 2.135 | 2.058 | 2.864 | 2.758 | 2.755 | 2.740 | 2.772 |
| Medial orbitofrontal | 2.519 | 2.432 | 2.382 | 2.500 | 2.430 | 3.076 | 2.959 | 2.939 | 2.974 | 2.969 |
| Middle temporal | 2.892 | 2.773 | 2.729 | 2.773 | 2.815 | 2.068 | 1.986 | 1.966 | 1.983 | 2.006 |
| Parahippocampal | 2.623 | 2.491 | 2.419 | 2.513 | 2.545 | 2.920 | 2.824 | 2.816 | 2.800 | 2.849 |
| Paracentral | 2.433 | 2.356 | 2.314 | 2.384 | 2.376 | 2.003 | 1.941 | 1.877 | 1.958 | 1.989 |
| Pars opercularis | 2.723 | 2.592 | 2.567 | 2.594 | 2.615 | 2.850 | 2.732 | 2.754 | 2.685 | 2.744 |
| Pars orbitalis | 3.007 | 2.890 | 2.903 | 2.853 | 2.902 | 2.757 | 2.697 | 2.709 | 2.658 | 2.712 |
| Pars triangularis | 2.673 | 2.558 | 2.561 | 2.581 | 2.538 | 2.739 | 2.642 | 2.645 | 2.642 | 2.640 |
| Pericalcarine | 1.711 | 1.693 | 1.667 | 1.753 | 1.675 | 2.709 | 2.604 | 2.615 | 2.605 | 2.592 |
| Postcentral | 2.094 | 2.002 | 1.971 | 2.023 | 2.017 | 2.312 | 2.247 | 2.214 | 2.279 | 2.254 |
| Posterior cingulate | 2.819 | 2.738 | 2.722 | 2.778 | 2.726 | 2.708 | 2.594 | 2.553 | 2.595 | 2.633 |
| Precentral | 2.444 | 2.329 | 2.300 | 2.343 | 2.346 | 2.643 | 2.553 | 2.514 | 2.577 | 2.572 |
| Precuneus | 2.440 | 2.344 | 2.311 | 2.406 | 2.332 | 2.525 | 2.443 | 2.417 | 2.463 | 2.455 |
| Rostral anterior cingulate | 2.868 | 2.759 | 2.717 | 2.807 | 2.765 | 2.840 | 2.682 | 2.634 | 2.700 | 2.716 |
| Rostral middle frontal | 2.576 | 2.477 | 2.481 | 2.467 | 2.481 | 2.678 | 2.582 | 2.568 | 2.569 | 2.605 |
| Superior frontal | 2.970 | 2.884 | 2.848 | 2.908 | 2.900 | 2.579 | 2.467 | 2.449 | 2.468 | 2.483 |
| Superior parietal | 2.202 | 2.131 | 2.116 | 2.160 | 2.124 | 2.258 | 2.200 | 2.157 | 2.227 | 2.221 |
| Superior temporal | 2.866 | 2.742 | 2.728 | 2.728 | 2.766 | 1.285 | 1.217 | 1.177 | 1.232 | 1.244 |
| Supramarginal | 2.653 | 2.541 | 2.481 | 2.564 | 2.580 | 2.423 | 2.352 | 2.343 | 2.380 | 2.341 |
| Frontal pole | 3.010 | 2.944 | 2.863 | 3.020 | 2.968 | 2.922 | 2.807 | 2.814 | 2.782 | 2.818 |
| Temporal pole | 3.743 | 3.685 | 3.623 | 3.637 | 3.777 | 2.576 | 2.458 | 2.312 | 2.436 | 2.612 |
| Transverse temporal | 2.382 | 2.263 | 2.223 | 2.303 | 2.272 | 0.024 | 0.037 | 0.040 | 0.035 | 0.036 |
a. Results in bold indicate values significantly different (compared with the control group) at a threshold of P < 0.05, false discovery rate-adjusted.
Vertex-wise analyses showed a similar pattern, with the negative symptoms subgroup showing the most extensive deficits (Fig. 1). Regions of overlap of the three subgroups included left superior and inferior frontal, right superior temporal cortical areas.

Fig. 1 Vertex-wise analysis (P<0.05, false discovery rate-adjusted) of cortical thickness and statistical comparison (v. control group) of the entire schizophrenia group (left column), the negative symptoms subgroup (second column), disorganised symptoms subgroup (third column) and paranoid symptoms subgroup (last column).
Discussion
Schizophrenia subgroups
Before their removal in DSM-5, 33 clinical subtypes of schizophrenia have been a consistent feature of diagnostic classification; however, their application in biological research has seen gradual decline. Reference Tandon, Gaebel, Barch, Bustillo, Gur and Heckers13,Reference Braff, Ryan, Rissling and Carpenter34 This is at odds with the widely accepted notion that the (wider) schizophrenia definition most likely encompasses several biologically distinct disease entities. Identification of putative biological markers for schizophrenia will therefore have to consider not only variability introduced by general factors (such as disease onset, disease severity and so on), but also whether this marker relates to a common deficit or to a pathophysiological feature expressed in varying degrees across subgroups.
In this study, we provide evidence that regional variability of cortical thinning in schizophrenia is related to symptom profiles and distinct subgroups. Our findings add to an increasing body
of research highlighting the biological heterogeneity of schizophrenia, which could provide important hints to delineating universal v. specific pathological pathways. Reference Takayanagi, Wentz, Takayanagi, Schretlen, Ceyhan and Wang22,Reference Voineskos, Foussias, Lerch, Felsky, Remington and Rajji35
Recent studies have established that regional cortical thinning is not only present at disease onset in schizophrenia, but also is evident in high-risk individuals in some areas. Reference Sprooten, Papmeyer, Smyth, Vincenz, Honold and Conlon4 In addition, several recent studies have addressed the influence of certain clinical factors of disease and genetic risk on cortical thinning in schizophrenia. Reference Bergmann, Haukvik, Brown, Rimol, Hartberg and Athanasiu16,Reference Schultz, Nenadic, Koch, Wagner, Roebel and Schachtzabel18,Reference Assuncao Leme, Gadelha, Sato, Kiyomi Ota, de Jesus Mari and Melaragno36 Yang e t al have demonstrated that cortical thinning might also be observed in non-psychotic relatives of people with schizophrenia, although those with the disease had more pronounced thinning, especially in frontal and temporal areas. Reference Yang, Nuechterlein, Phillips, Hamilton, Subotnik and Asarnow12 Although this establishes both alterations related to disease liability or general phenotypic variation, there is rather little data on the heterogeneity of cortical thickness within participant groups with schizophrenia that would reflect possible subgroups within the disease entity. There is recent evidence of an association between the deficit subtype of schizophrenia and regional cortical thinning, but the results have been mixed. Whereas one found a significant difference in anterior cingulate thickness and volume, Reference Takayanagi, Wentz, Takayanagi, Schretlen, Ceyhan and Wang22 another study found mostly overlapping regional thinning in patients with and without deficits compared with a control group. Reference Voineskos, Foussias, Lerch, Felsky, Remington and Rajji35 Therefore, cortical thickness may be unable to separate those subtypes, albeit other morphometric markers did diverge.
Compared with the subtypes used in our study, the deficit syndrome relies mostly on outcome, i.e. longitudinal aspects. Although this might result in higher temporal stability of subgrouping, it remains to be clarified whether the deficit subtype truly correlates with a distinct biological endophenotype or merely represents a more severe disease form. Before the introduction of DSM-5, schizophrenia subtypes in DSM-III-R 37 and DSM-IV 24 have been based on clinical prototypes rather than biological parameters. Their validity on biological grounds has been insufficiently supported by (biological) research. Reference Tandon, Gaebel, Barch, Bustillo, Gur and Heckers13 Although our approach has also been based on psychopathology as a clinical phenotype, it has not utilised prototypes or assignment to a subgroup by clinical decision, but rather has used psychometric aspects of symptom ratings to develop a more stable clinical phenotype, which is less susceptible to clinical rating biases.
Differences in our findings between subgroups
The regional patterns in frontal, temporal and cingulate areas across our three subsamples merit some attention. Our findings in the negative symptoms subgroup most closely resemble a pattern of widespread cortical thinning, almost lacking regional specificity, as seen in some recent large samples. Reference Rimol, Nesvag, Hagler, Bergmann, Fennema-Notestine and Hartberg6 Probably the greatest overlap between our three subgroups was seen in prefrontal regions, where all three groups showed convergence in areas covering both the orbitofrontal cortices (except left medial orbitofrontal cortex for the disorganised symptoms subgroup, which did not reach significance), superior and rostral middle frontal (again, except left superior frontal cortex for the disorganised symptoms subgroup) and right frontal pole regions. In contrast, temporal areas, such as the temporal pole, fusiform cortex and even superior temporal showed less convergence, as none of these areas showed thinning across all three groups. This was also the case for the medial temporal areas (parahippocampal and entorhinal). Our study did not include a detailed analysis of hippocampal thickness, which requires other analytical approaches to address the more convoluted topography. Reference Brambilla, Perlini, Rajagopalan, Saharan, Rambaldelli and Bellani38
Considering the three subgroups in terms of how many regions showed reduction of cortical thickness, it is noteworthy that the disorganised symptoms subgroup showed the least deficits, which is consistent with recent VBM studies, where the extent of deficits was also smallest in this subgroup. Reference Koutsouleris, Gaser, Jager, Bottlender, Frodl and Holzinger19,Reference Nenadic, Sauer and Gaser20 Overall, the pattern of the negative symptoms subgroup showing the most widespread pathology is consistent with our previous study assessing cortical complexity, Reference Nenadic, Yotter, Sauer and Gaser21 although cortical complexity variation (both reduction and increase) in the negative symptoms subgroup was not as widespread and extensive as the cortical thickness deficit.
Limitations
The study has some limitations. Given that this is a cross-sectional study with patients well past their first episode, the effects of disease progression, which might diverge between subgroups, Reference Nenadic, Sauer, Smesny and Gaser39 cannot be controlled for. Subgroups might also have varied disease severity, which is difficult to assess, especially in the absence of unequivocal unidimensional markers. This is also the case for antipsychotic medication, which might at least have some subtle effects on cortical thickness (including frontal areas), although recent studies in psychosis are not suggestive of very strong effects Reference Roiz-Santianez, Tordesillas-Gutierrez, Ortiz-Garcia de la Foz, Ayesa-Arriola, Gutierrez and Tabares-Seisdedos40 and actually demonstrate some reversibility of cortical thinning during short-term treatment. Reference Goghari, Smith, Honer, Kopala, Thornton and Su41 In participants with chronic schizophrenia, cumulative intake of antipsychotics is often difficult to assess, and given potential non-adherence to treatment, is also difficult to assess retrospectively. Chlorpromazine equivalents, which are often used in clinical studies to calculate simple dose equivalents between different antipsychotics, are likely not to be useful for this type of study, since brain structural effects of antipsychotics are probably not only related to dopamine D2 receptor occupancy or other pharmacological factors important for assessment of clinical efficacy. Finally, we also cannot rule out effects of alcohol intake or illicit substances. These effects are difficult to track, especially when assessed retrospectively.
Implications
We conclude from our findings, that although cortical thickness is reduced in schizophrenia across multiple areas, especially the prefrontal cortex, the spatial extent and effect size are variable across subgroups delineated by predominant symptoms. Our results warrant further exploration of the notion that different patterns of regional thinning might be related to different biological subtypes, which might then be used as different intermediate phenotypes for the dissociation of genetically distinct forms of the disease.
Funding
This study was supported by grants from the Friedrich-Schiller-University of Jena (DRM 21007087 to I.N.) and the BMBF (BMBF 01EV0709 and BMBF01GW0740 to C.G.).








eLetters
No eLetters have been published for this article.