More than three decades ago vulnerability–stress models emphasised that psychotic symptoms stem from an interaction of individual vulnerability and experienced stressors. Reference Nuechterlein and Dawson1,Reference Zubin and Spring2 Since then, research has broadened our understanding of vulnerability, important stressors and possible mechanisms of their interaction to elucidate the development and maintenance of psychotic disorders such as schizophrenia. Reference Van Os, Kenis and Rutten3 Autonomic dysfunction is emphasised as a core feature of the models, linking vulnerability to the everyday experience of stressors, due to an impaired adaptation to environmental challenges. Reference Nuechterlein and Dawson1 Indeed, physiological measures that reflect autonomic function may serve as indices of the extent to which an individual is able to flexibly and adaptively regulate emotional, behavioural and physiological responses when facing changing environmental conditions. Reference Thayer, Ahs, Fredrikson, Sollers and Wager4 Thus, the autonomic nervous system appears a promising target for schizophrenia research.
Both branches of the autonomic nervous system – the sympathetic and the parasympathetic – dually innervate the heart. Parasympathetic vagal activity decelerates the heart rate whereas sympathetic activity accelerates it, not only in response to environmental demands but also in relation to bodily signals such as respiration and the baroreflex. The resulting variability in the heart rate serves as an important marker of autonomic nervous system activity and of functional connectivity in related areas of the brain. The model of neurovisceral integration proposes that the heart rate variability (HRV) serves as a readily available index of central–peripheral neural feedback mechanisms, highlighting cardiac vagal tone as a psychophysiological resource when facing environmental challenges. Reference Thayer and Lane5 Low cardiac vagal activity (resulting in low HRV), for example due to a constant perception of threat, leads to a lack of highly necessary recreational phases.
Methodologically the HRV is derived from the inter-beat interval time series, reflecting time intervals between adjacent heartbeats in milliseconds. Numerous methods of operationalising HRV exist but fall broadly into one of the three classes of time domain, frequency domain and non-linear measures. This meta-analysis considered time and frequency domain measures, as those are most frequently applied and reported most consistently. A precise overview of different measures and underlying mechanisms may be found elsewhere. 6 Time domain indices are derived directly from the inter-beat interval series and generally measure the variability contained therein. Frequency domain measures are derived through spectral analytic techniques such as fast Fourier transform or autoregressive algorithm applied to the inter-beat interval series. The power spectrum of short-term HRV recordings contains two major components: high frequency (0.15–0.40 Hz) and low frequency (0.01–0.15 Hz). Parasympathetic modulation of the heart rate is fast (milliseconds) whereas sympathetic effects are much slower. Reference Levy7 Thus, time and frequency domain measures reflecting these fast changes – the root mean square of successive R–R interval differences (RMSSD) and high-frequency HRV – index vagal parasympathetic activity.
Several commonalities are evident in the research conducted on schizophrenia and HRV and are worth mentioning. First, schizophrenia has been associated with an increased risk of cardiovascular disease as well as cardiac mortality, Reference Hennekens, Hennekens, Hollar and Casey8 and HRV has been found to be a reliable indicator of such risk. 6 Second, complex executive dysfunctions are reported in individuals with schizophrenia; Reference Neill and Rossell9 HRV is highly relevant for these functions. Reference Thayer, Hansen, Saus-Rose and Johnsen10,Reference Hansen, Johnsen and Thayer11 Third, difficulties in emotion regulation are prominent in schizophrenia, Reference Lincoln, Hartmann, Kother and Moritz12 which are also associated with decreased HRV. Reference Thayer and Lane5 Finally, studies that investigated neural factors underlying the development of schizophrenia identified several brain regions that differed in structure or functionality compared with healthy individuals. These regions included different areas of the anterior cingulate cortex and the medial prefrontal cortex, Reference Jacobson McEwen, Connolly, Kelly, Kelleher, O'Hanlon and Clarke13–Reference Williams, Das, Harris, Liddell, Brammer and Olivieri15 regions in which activity has also been associated with HRV. Reference Thayer, Ahs, Fredrikson, Sollers and Wager4,Reference Thayer, Hansen, Saus-Rose and Johnsen10 Reduced HRV could thus provide an endophenotype for schizophrenia, Reference Castro, Vigo, Weidema, Fahrer, Chu and de Achaval16,Reference Castro, Vigo, Chu, Fahrer, de Achaval and Costanzo17 characterised by an increased risk of cardiovascular disease, difficulties in executive functioning, emotion regulation and disinhibition that link it to the development and maintenance of psychotic symptoms. This endophenotype could serve as an important target in both the prevention and treatment of schizophrenia and provide a valuable biomarker for research.
Studies have shown that vagal activity during rest is significantly decreased in unmedicated patients with acute schizophrenia compared with healthy control groups. Reference Bar, Letzsch, Jochum, Wagner, Greiner and Sauer18,Reference Bar, Rachow, Schulz, Bassarab, Haufe and Berger19 There is also evidence that although individuals with schizophrenia show a similar adaptation to a stressor, they exhibit lower HRV in the following recovery period compared with a control group. Reference Castro, Vigo, Weidema, Fahrer, Chu and de Achaval16 As anticholinergic effects are reported for antipsychotic drugs, effects of medication on HRV in schizophrenia have been specifically addressed. It was shown that medicated patients with schizophrenia show lower vagal activity than healthy individuals, Reference Castro, Vigo, Weidema, Fahrer, Chu and de Achaval16,Reference Kim, Ann and Lee20 and that some types of medication may cause more deterioration than others. Reference Agelink, Majewski, Wurthmann, Lukas, Ullrich and Linka21 However, follow-up assessments from an unmedicated to a medicated status imply that decreased vagal activity in schizophrenia deserves consideration beyond simple effects of medication intake. Reference Bar, Letzsch, Jochum, Wagner, Greiner and Sauer18 Most interestingly, patterns of HRV alteration similar to those found in schizophrenia are reported in healthy first-degree relatives, Reference Castro, Vigo, Chu, Fahrer, de Achaval and Costanzo17,Reference Bar, Rachow, Schulz, Bassarab, Haufe and Berger19 and also in people with prodromal symptoms, Reference Valkonen-Korhonen, Tarvainen, Ranta-Aho, Karjalainen, Partanen and Karhu22 which lends support to the notion of a potential endophenotype. Nevertheless, several other participant characteristics have been discussed to moderate autonomic nervous system alterations. Generally, HRV has been found to decrease with age; Reference Zhang23 furthermore, previous schizophrenia research has indicated a possible association of decreased HRV with increased symptom severity, Reference Kim, Ann and Lee20,Reference Bar, Wernich, Boettger, Cordes, Boettger and Loffler24 and with the duration of disease. Reference Bar, Boettger, Berger, Baier, Sauer and Yeragani25 Hence, the heterogeneity of investigated indices, of participants and of results, Reference Lee, Park, Choi and Park26 question the robustness and the size of the effect. We therefore aimed to summarise the current evidence concerning HRV differences in individuals with schizophrenia compared with healthy control groups. In particular, given the aforementioned importance of vagal activity, we focused on quantifying time and frequency domain HRV indices reflecting parasympathetic influences. Time and frequency domain measures were considered, as these are most frequently applied and most likely to be reported consistently across primary studies. Furthermore, possible covariates were subjected to meta-regressions and subgroup analyses to explore clinical and methodological heterogeneity. To our knowledge, this is the first attempt to condense the existing research on HRV and schizophrenia.
Method
In August and September 2014 we conducted a systematic literature search in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, Reference Moher, Liberati, Tetzlaff and Altman27 in seven computerised databases (PubMed, Medline, PSYNDEX, Embase, PsycINFO, Web of Science and CINAHL). The search terms were ‘(“heart rate variability” OR “HRV”) AND (“schizophreni*” OR “psychosis”)’ and were limited to the abstract if possible. Additionally, the reference lists of all screened full-text papers were searched for further references of interest (related terms in titles, e.g. ‘variability’, ‘autonomic’, ‘schizophrenia’ and ‘psychosis’). After removing duplicate findings, the abstracts of all studies were screened based on pre-defined criteria. To be included, a study had to be (a) a peer-reviewed, empirical investigation, (b) written in English or German, (c) reporting HRV, (d) conducted on participants with schizophrenia or another psychotic disorders, and (e) on a healthy control group.
Data extraction and quality assessment
The following data were extracted from the included studies and coded: year of publication, language, country of research origin and main study focus. Information on study samples was obtained regarding the total sample size, size of included groups, age, gender distribution, the diagnostic criteria and sample nature and recruitment of the schizophrenia and control groups. Finally, we coded details of the HRV recording providing information on the technique of assessment (e.g. electrocardiogram, ECG), sample rate, condition at recording (e.g. supine), recording length of interest, derived HRV indices and reported units.
Measures of HRV reflecting vagal activity were selected based on the guidelines of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. 6 For this meta-analysis we included components that have been considered to reflect primarily vagal cardiac modulation only, as these are more readily interpretable and because vagal activity seems to have a unique role in patients with schizophrenia. Reference Bar, Letzsch, Jochum, Wagner, Greiner and Sauer18 Thus, studies that reported (or authors who provided) RMSSD or any spectral measure in the high-frequency range of 0.15–0.40 Hz were included. If more than one unit of high frequency was reported, we analysed the standardised values (i.e. normalised, in percentages). Means and standard deviations of these measures were extracted, or requested when insufficient quantitative data were available. When only the standard error of the mean (SEM) was reported, the standard deviation was calculated by multiplying the SEM by the square root of the sample size. Reference Higgins and Green28 If the given means were estimates of covariance analyses, the corresponding authors were asked to provide raw means. If 24 h long-term measurements were available, these were included. Otherwise, recordings obtained from resting or baseline conditions were extracted. When only day or night values were available, the night values were analysed as they are more likely to reflect vagal activity owing to less movement and other disturbing influences. Furthermore, authors with potential access to data of interest (i.e. reporting measurement of HRV in schizophrenia and control groups but no data on group analyses; reporting non-linear results or measures of the QT interval only) were contacted.
In several studies the schizophrenia group was stratified into different subgroups (e.g. according to type of medication). If one of these subgroups was an unmedicated group it was selected for data extraction in order to analyse the effects of schizophrenia itself rather than effects related to medication intake. In other cases, data for the total group were requested from the authors. If these values were not available from the authors we combined the groups according to the Cochrane Handbook. Reference Higgins and Green28 All data extraction was performed and checked by A.C. and supervised by J.K. If data or methods were ambiguous the content was discussed until A.C. and J.K. reached consensus.
Statistical analysis
Effect size estimation
To estimate the true effect size across the different studies with variance in reported units, standardised mean differences (SMD; Hedges' g) with 95% confidence intervals were calculated. The random effects model was applied. In four cases, no data were retrieved on standard deviations; however, range, interquartile range or post-hoc t-test values were available. According to the Cochrane guidelines, standard deviations may be estimated from these. Reference Higgins and Green28 However, as this is not strongly recommended and because HRV data are potentially skewed, additional secondary analyses were conducted excluding studies with estimated values. Statistical heterogeneity was tested with the standard I 2 index, χ2-tests and τ2-tests. Reference Higgins and Thompson29 A possible reporting or publication bias was examined using a funnel plot, depicting the effect size against the standard error for asymmetry. All meta-analytic computations were performed with the RevMan software version 5.3.4 (Cochrane Collaboration).
Meta-regression and subgroup analyses
To explore the potential effect of trial-level modifiers, we considered several covariates in meta-regression approaches, conducted with a single covariate at a time. Reference Knapp and Hartung30 Meta-regression allows the investigation of the effect of continuous and categorical characteristics. Reference Higgins and Green28 We applied the random effects model which acknowledges that some of the heterogeneity of the effects might not be modelled by the covariates. Reference Higgins and Green28 Because some of the defined variables of interest were associated with a small number of studies, subgroup analyses were conducted if fewer than ten studies were available for a category. Meta-regressions and subgroup analyses were conducted using OpenMetaAnalyst software. Reference Wallace, Dahabreh, Trikalinos, Lau, Trow and Schmid31 To account for clinical heterogeneity, four population-level covariates were defined for the schizophrenia patient groups: mean age, mean duration of disease, medication (dichotomously coded as medicated v. unmedicated) and clinical category of recruited sample (e.g. out-patients). No direct measure of symptom severity could be included because methods of assessment varied greatly. Instead, the patient samples were categorised by best fit into the clinical categories ‘in-patients/ hospitalised’ or ‘out-patients’ and ‘first episode in-patients/ hospitalised’ or ‘chronic’. To account for methodological heterogeneity, three covariates regarding the HRV measurements were included: recording length – short (< 1 h) or long (⩾ 1 h), method of assessment (ECG or photoplethysmography) and the unit of reported high-frequency values (i.e. absolute power or normalised in proportion to total frequency).
Results
Initially 505 studies were retrieved from the selected databases (Fig. 1). In addition, 34 studies were identified by searching the reference lists of all subsequently included full-text articles. After removal of duplicates 262 abstracts were systematically screened for inclusion based on the pre-defined criteria, leaving 56 papers potentially eligible for inclusion. Two requests for full-text papers were not answered, and data were extracted from the remaining 54 full-text papers. Of these, 31 studies reported insufficient values (e.g. range instead of standard deviations, means and standard deviations in figures only) and the corresponding authors were contacted to request total or partial data. Finally, the studies were subjected to meta-analysis if effect sizes could be calculated or estimated from the available data (k = 34). For 19 of the included studies both high-frequency HRV and RMSSD were available as outcomes; Reference Castro, Vigo, Weidema, Fahrer, Chu and de Achaval16,Reference Bar, Letzsch, Jochum, Wagner, Greiner and Sauer18,Reference Agelink, Majewski, Wurthmann, Lukas, Ullrich and Linka21,Reference Valkonen-Korhonen, Tarvainen, Ranta-Aho, Karjalainen, Partanen and Karhu22,Reference Bar, Wernich, Boettger, Cordes, Boettger and Loffler24,Reference Lee, Park, Choi and Park26,Reference Agelink, Zeit, Baumann, Majewski, Lemmer and Postert32–Reference Rachow, Berger, Boettger, Schulz, Guinjoan and Yeragani44 for 10 studies only high-frequency data were available, Reference Boettger, Hoyer, Falkenhahn, Kaatz, Yeragani and Bar45–Reference Mujica-Parodi, Yeragani and Malaspina54 and for 5 studies only RMSSD data were available. Reference Kim, Ann and Lee20,Reference Bar, Boettger, Berger, Baier, Sauer and Yeragani25,Reference Birkhofer, Geissendoerfer, Alger, Mueller, Rentrop and Strubel55–Reference Toichi, Kubota, Murai, Kamio, Sakihama and Toriuchi57

Fig. 1 Study flow chart. HRV, heart rate variability; RMSSD, root mean square of successive R–R interval differences.
Study and sample characteristics
We included data from 3055 participants for meta-analysis of high-frequency HRV and from 2485 participants for meta-analysis of RMSSD. Detailed characteristics of the included studies and their samples can be found in online Table DS1. The averaged mean age of participants with schizophrenia was 36.8 years, with a range of means from 21.2 years to 56.2 years. In studies that reported a mean duration of illness (k = 19) the overall mean duration was 9.9 years (range 3–22.7). Fifteen studies were conducted in participants not receiving medication (44%) and the other 19 (56%) in individuals medicated with varying types of antipsychotics. Of the studies that defined a specific schizophrenia sample for recruitment, 3 studies reported data on chronic schizophrenia (9%), 17 on in-patients (50%, including 2 studies specifically on in-patients with first-episode disorder) and 5 on out-patients (15%). Twenty-nine of the included studies used ECG (85%) and 3 used photoplethysmography (9%) to measure HRV. In 28 studies (82%) assessment was short-term whereas in 4 (12%) assessment was considered as long-term (i.e. more than 1 h). Of the 29 studies with information on high-frequency HRV, 6 studies provided the data as normalised power (18%). In 21 cases the reported units reflected absolute power (62%). Detailed study characteristics on HRV assessment are provided in online Table DS2.
Main effects
High-frequency variability
The meta-analysis revealed a significant effect (Z = 3.35, P = 0.0008), indicating that individuals with schizophrenia (n = 1353) have lower high-frequency HRV compared with healthy controls (n = 1702; g = −0.98, 95% CI −1.56 to −0.41; k = 29). Tests for statistical heterogeneity revealed significance (Fig. 2), indicating a possible bias. Visual inspection of the funnel plot showed that the reported effect was mainly based on larger studies reporting a significant effect, whereas a few smaller and non-significant studies appeared to be lacking (Fig. 3). In secondary analyses to assess this possible bias, we evaluated the main effect after excluding the most extreme SMDs (i.e. > 2), but still found a significant effect of similar size (Z = 8.38, P<0.0001; g = −0.87, 95% CI −1.07 to −0.67; k = 26). Because the estimation of standard deviations from range or interquartile range is not recommended by the Cochrane guidelines, we also calculated the effect omitting the studies that lacked information on standard deviations and potentially included estimated means. Again, a significant effect of a similar size was evident (Z = 2.9, P = 0.003; g = −0.93, 95% CI −1.54 to −0.32; k = 26). The forest plots for these secondary analyses can be found in online Fig. DS1.

Fig. 2 Meta-analysis of main effects: high-frequency domain heart rate variability. SMD, standardised mean difference.

Fig. 3 Funnel plot of main effects: high-frequency heart rate variability. SE, standard error; SMD, standardised mean difference.
RMSSD
The meta-analysis with RMSSD as the dependent variable also revealed a significant effect (Z = 6.18, P<0.0001), indicating that individuals with schizophrenia (n = 1016) have lower RMSSD compared with healthy controls (n = 1469; g = −0.91, 95% CI −1.19 to −0.62; k = 24). For details of study data and SMDs see Fig. 4. Tests for statistical heterogeneity revealed strong evidence of possible bias (Fig. 4); again, visual inspection of the funnel plot suggested that the reported effect was mainly based on larger studies reporting a significant effect, whereas a few smaller and non-significant studies were missing (Fig. 5). In the secondary analyses to examine possible bias, we analysed the main effect excluding studies with extreme SMDs (i.e. >2); the meta-analysis still revealed a significant effect (Z = 5.83, P<0.0001; g = −0.69, 95% CI −0.92 to −0.46; k = 22). We repeated the calculation excluding studies without reported standard deviations, and again found the significant effect indicating decreased vagal activity in schizophrenia was robust (Z = 5.75, P<0.0001; g = −0.90, 95% CI −1.21 to −0.60; k = 21). The forest plots for these secondary analyses are provided in online Fig. DS2.

Fig. 4 Meta-analysis of main effects: root mean square of successive R–R interval differences (RMSSD). SMD, standardised mean difference.
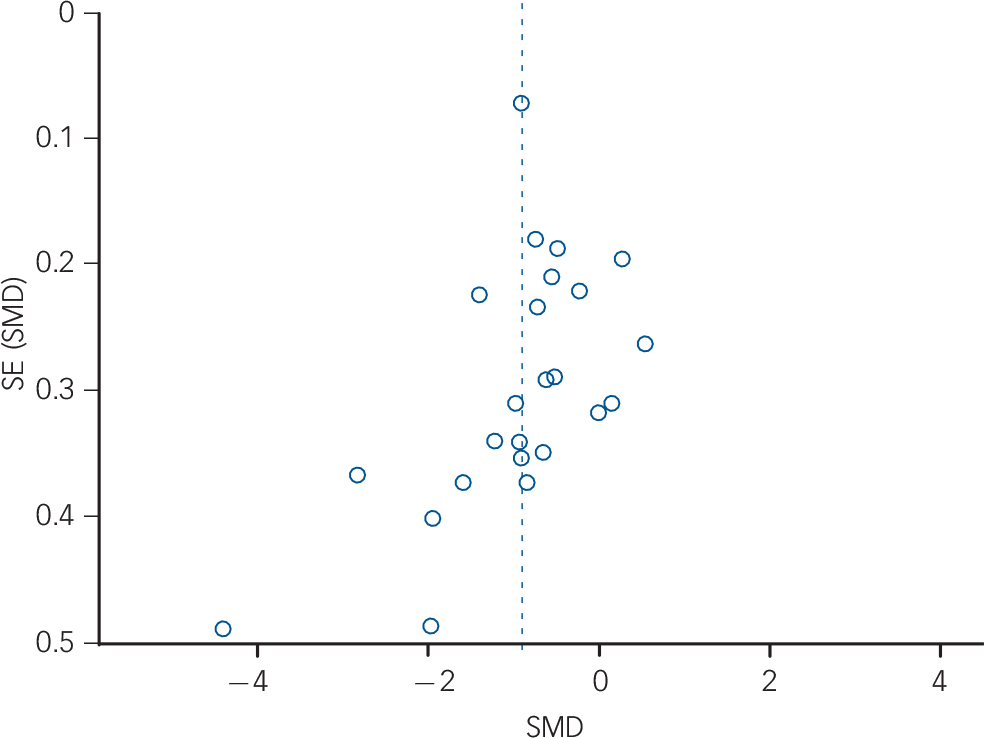
Fig. 5 Funnel plot of main effects: root mean square of successive R–R interval differences. SE, standard error; SMD, standardised mean difference.
Meta-regressions and subgroup analyses
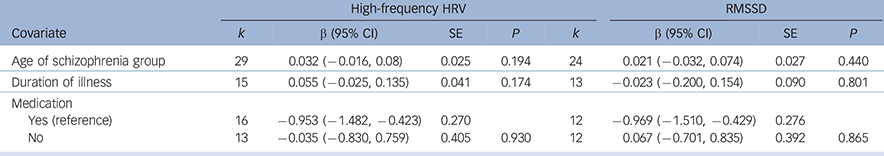
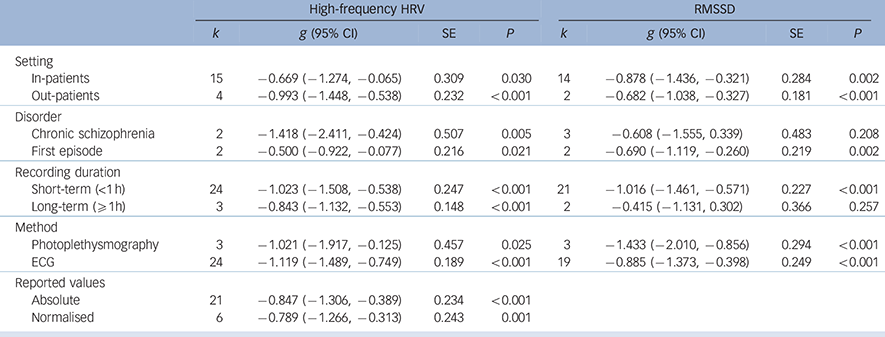
Analyses of study-level covariates showed no significant effect as a function of age, duration of illness or medication (Table 1). In a subgroup analysis we compared the effect of studies that investigated in-patients with those investigating out-patients (Table 2). Again, no difference was found, with both groups showing a significant effect of similar magnitude, indicating lower high-frequency HRV and RMSSD in both schizophrenia groups compared with controls. When comparing the chronic schizophrenia group with first-episode patients, a slightly different picture was revealed: for high-frequency HRV both groups showed a significant main effect; however, the effect for patients with chronic schizophrenia was large whereas the effect for first-episode patients was medium in size. In contrast, the effect for RMSSD was not significant for patients with chronic schizophrenia, but was significant for first-episode patients. It is important to note the relatively large SE for the group with chronic schizophrenia (Table 2). Analyses of methodological heterogeneity showed that all but one HRV measurement subgroup showed a significant effect. Thus, the schizophrenia group evidenced lower vagal activity than controls for both outcome variables regardless of the applied methods, with one exception: no significant effect was found when only studies of long-term recordings of RMSSD were analysed. Again, it is noteworthy that the SE was relatively large and the analysis was based on two studies (see Table 2 for all effects and estimated P-values).
Table 1 Meta-regression of pre-defined variables of interest
| High-frequency HRV | RMSSD | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | k | β (95% CI) | SE | P | k | β (95% CI) | SE | P |
| Age of schizophrenia group | 29 | 0.032 (−0.016, 0.08) | 0.025 | 0.194 | 24 | 0.021 (−0.032, 0.074) | 0.027 | 0.440 |
| Duration of illness | 15 | 0.055 (−0.025, 0.135) | 0.041 | 0.174 | 13 | −0.023 (−0.200, 0.154) | 0.090 | 0.801 |
| Medication | ||||||||
| Yes (reference) | 16 | −0.953 (−1.482, −0.423) | 0.270 | 12 | −0.969 (−1.510, −0.429) | 0.276 | ||
| No | 13 | −0.035 (−0.830, 0.759) | 0.405 | 0.930 | 12 | 0.067 (−0.701, 0.835) | 0.392 | 0.865 |
HRV, heart rate variability; RMSSD, root mean square of successive R-R-interval differences; SE, standard error.
Table 2 Subgroup analyses of pre-defined variables of interest
| High-frequency HRV | RMSSD | |||||||
|---|---|---|---|---|---|---|---|---|
| k | g (95% CI) | SE | P | k | g (95% CI) | SE | P | |
| Setting | ||||||||
| In-patients | 15 | −0.669 (−1.274, −0.065) | 0.309 | 0.030 | 14 | −0.878 (−1.436, −0.321) | 0.284 | 0.002 |
| Out-patients | 4 | −0.993 (−1.448, −0.538) | 0.232 | <0.001 | 2 | −0.682 (−1.038, −0.327) | 0.181 | <0.001 |
| Disorder | ||||||||
| Chronic schizophrenia | 2 | −1.418 (−2.411, −0.424) | 0.507 | 0.005 | 3 | −0.608 (−1.555, 0.339) | 0.483 | 0.208 |
| First episode | 2 | −0.500 (−0.922, −0.077) | 0.216 | 0.021 | 2 | −0.690 (−1.119, −0.260) | 0.219 | 0.002 |
| Recording duration | ||||||||
| Short-term (<1 h) | 24 | −1.023 (−1.508, −0.538) | 0.247 | <0.001 | 21 | −1.016 (−1.461, −0.571) | 0.227 | <0.001 |
| Long-term (⩾ 1h) | 3 | −0.843 (−1.132, −0.553) | 0.148 | <0.001 | 2 | −0.415 (−1.131, 0.302) | 0.366 | 0.257 |
| Method | ||||||||
| Photoplethysmography | 3 | −1.021 (−1.917, −0.125) | 0.457 | 0.025 | 3 | −1.433 (−2.010, −0.856) | 0.294 | <0.001 |
| ECG | 24 | −1.119 (−1.489, −0.749) | 0.189 | <0.001 | 19 | −0.885 (−1.373, −0.398) | 0.249 | <0.001 |
| Reported values | ||||||||
| Absolute | 21 | −0.847 (−1.306, −0.389) | 0.234 | <0.001 | ||||
| Normalised | 6 | −0.789 (−1.266, −0.313) | 0.243 | 0.001 | ||||
ECG, electrocardiogram; HRV, heart rate variability; RMSSD, root mean square of successive R-R-interval differences; SE, standard error.
Discussion
This meta-analysis is the first to summarise the existing research on HRV in schizophrenia. Including data collected in more than a thousand individuals with schizophrenia compared with an equally large healthy control group, we were able to provide strong evidence that vagal activity is significantly reduced in schizophrenia. This finding is further corroborated by the fact that we found a distinct but similar effect for two different measures of vagal activity: the RMSSD and high-frequency power. For each outcome the effect was large – for both indices in the same magnitude – and remained stable within secondary analyses excluding studies that carried potential risk of bias.
Emphasising the robustness of our findings, we analysed differences in HRV in different subgroups of patients – such as those with chronic or first-episode schizophrenia, acute in-patient or stable out-patient treatment, and use of medication. Furthermore, the original research used in our analysis was conducted in various countries and within a variety of settings (Tables DS1 and DS2). As this was also notable in statistical heterogeneity, we conducted several additional analyses. In meta-regression analyses, potential variables to influence the selected dependent measures (RMSSD and high-frequency HRV) revealed no significant effect on the resting state vagal activity difference between the schizophrenia and control groups. Two subgroup analyses yielded possible differences for RMSSD: no significantly decreased vagal activity was found in the subgroup of patients with chronic schizophrenia and in long-term recordings when compared with controls. However, owing to the small number of studies, these analyses may lead to new hypotheses for future studies but are not reliable. Also, none of the covariates addressed influenced the results for either of the assessed indices of vagal activity. Interestingly, we did not find a direct bias due to medication status. In both medicated and unmedicated patient groups we found reduced vagal activity, arguing that the reported main effect is not related to the intake of antipsychotic medication. However, it is to be discussed critically that in all studies except for the two investigating first-episode patients, Reference Valkonen-Korhonen, Tarvainen, Ranta-Aho, Karjalainen, Partanen and Karhu22,Reference Jindal, Keshavan, Eklund, Stevens, Montrose and Yeragani40 at least some of the participants received antipsychotics some time before assessment. Adding to this, the wash-out period in some studies was short (i.e. 5–10 days), Reference Agelink, Majewski, Wurthmann, Lukas, Ullrich and Linka21,Reference Bar, Wernich, Boettger, Cordes, Boettger and Loffler24,Reference Agelink, Zeit, Baumann, Majewski, Lemmer and Postert32,Reference Birkhofer, Geissendoerfer, Alger, Mueller, Rentrop and Strubel55 and previous medication could still have influenced autonomic nervous system functioning. Nevertheless, even in the two studies with first-episode participants, at least a medium-sized effect was found, highlighting the value of HRV as an index of the dysfunction of brain–body integration beyond simple effects of medication. For future research, it has to be noted that different kinds of medication may have a distinct effect on HRV, Reference Cohen, Loewenthal, Matar and Kotler47 and the possible deteriorative effect on HRV after the onset of medication shown by some studies, such as that by Agelink et al, Reference Agelink, Majewski, Wurthmann, Lukas, Ullrich and Linka21 needs to be further investigated. Unfortunately, the current number of studies included for subsequent analysis makes it difficult to analyse these effects across studies or to conduct a meta-analysis of within-study effects.
Our findings have a wide range of important implications. Reduced HRV may be considered as an endophenotype that is independently associated with executive dysfunction, difficulties in emotion regulation and disinhibition in schizophrenia. Reference Thayer and Lane5,Reference Thayer, Hansen, Saus-Rose and Johnsen10,Reference Hansen, Johnsen and Thayer11 In line with the propositions by the model of neurovisceral integration, low cardiac vagal tone is an index of impaired central–peripheral neural feedback mechanisms that leads to a lack of psychophysiological resources when an individual is confronted with environmental challenges. Reference Thayer and Lane5 Threat circuits that integrate internal and external context may be disinhibited, Reference Thayer, Ahs, Fredrikson, Sollers and Wager4 leading to a constant perception of threat and thus fear and arousal. Reference Williams, Das, Harris, Liddell, Brammer and Olivieri15 Notably, recent data have provided the first evidence of an association of high resting HRV with enhanced safety learning and fear extinction. Reference Pappens, Schroijen, Sutterlin, Smets, Van den Bergh and Thayer58
Limitations
Our results need to be regarded in the light of the study's limitations. First, a large number of studies had to be excluded because of insufficient data and a lack of response by contacted authors. This holds potential bias for the effect size reported. However, all excluded studies reported lower vagal activity for the schizophrenia groups compared with healthy control groups, so their inclusion would not have changed the direction of the findings. Nevertheless, visual analysis of the funnel plots revealed a possible publication bias, with a lack of small studies showing an effect favouring lower vagal activity in individuals with schizophrenia. Thus, an overestimation of the effect is possible. Adding to this, the number of studies included in the meta-analysis was not large enough for some meta-regressions and thus the risk of bias needs to be explored further. Finally, the relation to subgroup characteristics was investigated across trials and not within trials, which may have led to ecological bias. Accordingly, we can only conclude that these variables did not play a role at study level, whereas the within-study association was not investigated in this meta-analysis and should be considered in future studies.
Future research
Future research should further investigate the impact of reduced vagal activity on symptoms and information processing in schizophrenia. In particular, the association of HRV, threat processing and anxiety seems a promising research target, given that HRV is reduced in anxiety disorders, Reference Chalmers, Quintana, Abbott and Kemp59 and in depression with comorbid anxiety to a larger extent than in depression alone. Reference Kemp, Quintana, Felmingham, Matthews and Jelinek60 In the light of specificity, our results allude to a large effect in schizophrenia, whereas previous meta-analyses suggested small to moderate effects in anxiety disorders and depression. Reference Chalmers, Quintana, Abbott and Kemp59,Reference Kemp, Quintana, Gray, Felmingham, Brown and Gatt61 However, direct comparisons are needed as existing studies across different disorders are rare and inconclusive. Reference Clamor, Hartmann, Kother, Otte, Moritz and Lincoln38,Reference Moon, Lee, Kim and Hwang42 Furthermore, longitudinal designs in schizophrenia research are needed to test the predictive value of HRV for symptom development from non-clinical or prodromal stages. Also, the impact for therapeutic outcome may be of interest, as cognitive–behavioural treatments have been found to enhance HRV in depression. Reference Kemp and Quintana62
To conclude, vagal activity is significantly reduced in patients with schizophrenia. Given its potential to indicate central–peripheral integration and the close association with schizophrenia-relevant brain regions, to threat processing, emotion regulation and executive functioning, HRV seems to be a promising endophenotype for schizophrenia research.
Acknowledgements
We are grateful to all authors who provided data and those who responded to our requests: R. Bodén, M. Castro, S. Guinjoan, R. Hempel, Y. S. Kim, D. Musselman, S. Thomas and J. H. M. Tulen.












eLetters
No eLetters have been published for this article.