Many medicines used to treat conditions prevalent in older people possess anticholinergic activity and they may produce central and peripheral side-effects – sedation, confusion, dry mouth, adverse dental outcomes and constipation. Reference Boustani, Campbell, Munger, Maidment and Fox1 The risk of adverse outcomes including admission to hospital and falls increases with increasing anticholinergic exposure. Reference Boustani, Campbell, Munger, Maidment and Fox1,Reference Smithard, Fox, Maidment, Katona and Boustani2 Frail, older people are particularly vulnerable to anticholinergic adverse effects because of the high probability of exposure to treat multiple conditions, and increased age-related sensitivity to anticholinergic-related cognitive adverse effects. Reference Campbell, Boustani, Limbil, Ott, Fox and Maidment3 Furthermore, medical problems prevalent in older people such as constipation, sleep difficulties and dementia may be worsened by use of anticholinergics. Reference Flacker, Cummings, Mach, Bettin, Kiely and Wei4 Consequently, anticholinergic medications are considered potentially inappropriate in older populations, particularly those with dementia who have limited cognitive reserve. Reference Campbell, Boustani, Limbil, Ott, Fox and Maidment3,Reference O'Mahony, O'Sullivan, Byrne, O'Connor, Ryan and Gallagher5 A systematic review examining associations between drugs with anticholinergic effects and adverse outcomes in older adults carried out by Ruxton and colleagues concluded that exposure to individual medicines with anticholinergic effects or increased overall anticholinergic exposure may increase risk of falls, cognitive impairment and all-cause mortality. Reference Ruxton, Woodman and Mangoni6 In those over 65 years of age one recent study has shown associations with dementia and cognitive impairment. Reference Gray, Anderson, Dublin, Hanlon, Hubbard and Walker7
People with intellectual disabilities continue to have a shorter life expectancy compared with the general population, are at increased risk of mortality from preventable or treatable illnesses, Reference Heslop, Blair, Fleming, Hoghton, Marriott and Russ8 and experience health inequities, including barriers to accessing primary care. Reference Buszewicz, Welch, Horsfall, Nazareth, Osborn and Hassiotis9 They experience up to 2.5 times the health problems Reference Haveman, Heller, Lee, Maaskant, Shooshtari and Strydom10 and higher incidence of morbidities such as dental disease, eye disease, epilepsy and dementia. Reference Haveman, Perry, Salvador-Carulla, Walsh, Kerr and Van Schrojenstein11,Reference McCarron, O'Dwyer, Burke, McGlinchey and McCallion12 Dual diagnosis is common, with one study reporting 41% of adults with intellectual disabilities with mental illness, Reference Cooper, Smiley, Morrison, Williamson and Allan13 which increases the likelihood of polypharmacy. Higher use of antipsychotics and other psychotropics prescribed to manage mental health conditions and challenging behaviours is a concern Reference Tyrer, Cooper and Hassiotis14–16 and may confer additional risk as organic brain dysfunction may lead to idiosyncratic responses to drugs. Reference Bhaumik and Branford17 Drug-induced anticholinergic activity is thought to be additive; the overall burden of anticholinergic drugs determining the risk of adverse effects. Reference Han, Agostini and Allore18 People with intellectual disabilities may be at additional risk of experiencing the ‘prescribing cascade’ as for example, the high prevalence of antipsychotic use may lead to the prescribing of anticholinergics for movement disorders (extrapyramidal symptoms; anatomical therapeutic chemical (ATC) N04A), a practice no longer recommended in older people. Reference O'Mahony, O'Sullivan, Byrne, O'Connor, Ryan and Gallagher5,Reference Ruxton, Woodman and Mangoni6 Anticholinergic effects may be misattributed to a consequence of the normal ageing process and drugs with anticholinergic properties may be a cause of unrecognised adverse drug reactions. Reference Mintzer and Burns19
People with intellectual disabilities receive a variety of different medicines with anticholinergic activity, and scales that capture cumulative burden are needed to stratify risk. The Anticholinergic Cognitive Burden (ACB) scale is one such scale that computes a total score of drugs to determine individual anticholinergic burden. Reference Boustani, Campbell, Munger, Maidment and Fox1 The ACB scale identifies the burden of anticholinergic negative effects on cognition of medications (prescribed and over the counter); drugs with no anticholinergic effects score 0, drugs with possible anticholinergic effects score 1, drugs with definite cognitive anticholinergic effects score 2 or 3. Reference Boustani, Campbell, Munger, Maidment and Fox1 In one study an ACB score of 5 or more was associated with an Mini-Mental State Examination (MMSE) score of one point lower compared with an ACB score of 0. Reference Fox, Richardson, Maidment, Savva, Matthews and Smithard20 It also found the largest effect on cognitive decline was observed in people with mild dementia (MMSE 26–30). The ACB scale has been demonstrated to have predictive validity, with higher ACB scores associated with adverse clinical outcomes. Reference Salahudeen, Hilmer and Nishtala21 Given evidence in the general older population of the risks associated with anticholinergic exposure and of frailty, cognitive decline and adverse effects, we hypothesised that adverse effects would be associated with exposure to a high anticholinergic load in older people with intellectual disabilities. Our objectives were: (a) to determine each individual's cumulative exposure to anticholinergic medications by using the ACB scale; (b) to describe the pattern of anticholinergic medication use in relation to demographic and clinical characteristics and the most frequently reported therapeutic classes contributing to the anticholinergic burden; (c) to examine factors associated with higher anticholinergic burden exposure; and (d) to explore the relationship between ACB scale scores and indicators of central and peripheral anticholinergic adverse effects.
Method
Study design
Medication data for this study was drawn from Wave 1 (2009/2010) of the Intellectual Disability Supplement to the Irish Longitudinal Study on Ageing (IDS-TILDA), which contains a nationally representative sample of 753 people with an intellectual disabilities, aged between 41 and 90 years (online Fig. DS1) Reference McCarron, Swinburne, Burke, McGlinchey, Mulryan and Andrews22 IDS-TILDA is a longitudinal study of older adults with intellectual disabilities and has been described in detail elsewhere. Reference McCarron, Swinburne, Burke, McGlinchey, Mulryan and Andrews22,Reference McCarron, Swinburne, Burke, McGlinchey, Carroll and McCallion23 Age 40 years and over was selected to reflect the lower longevity of people with intellectual disabilities and their earlier onset of chronic disease, for example dementia. Reference Janicki and Dalton24,Reference Lavin, McGuire and Hogan25 This would also ensure that there would be sufficient participants for future waves of data collection and provide opportunities to offer insights into ageing for those who may age prematurely. Everyone included in the study was registered on the Irish National Intellectual Disability Database, and therefore had an intellectual disability. The person's level of intellectual disabilities was checked and confirmed from case notes at the time of the face-to-face interview. The STROBE (The Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines for cross-sectional studies was used. Reference Vandenbroucke, von Elm, Altman, Gøtzsche, Mulrow and Pocock26 Ethical approval for the study was received from the Faculty of Health Sciences Trinity College Dublin and 138 intellectual disability service providers, and all participants and/or proxies, as appropriate, provided informed consent to partake in the study.
Medication exposure measures
Participants/proxies were asked ‘Can you tell me what medications (including prescribed and over-the-counter, herbal medicines) you take on a regular basis – like every day or every week?’ in the pre-interview questionnaire. Reference McCarron, Swinburne, Burke, McGlinchey, Mulryan and Andrews22 The pre-interview questionnaire was sent to participants and/or proxies 1 week in advance of the face-to-face interview to give them time to check patient records or charts to record the medicines they were taking. This information was then cross-checked by interviewers at the time of interview, by asking whether the list they had provided in the pre-interview questionnaire included all of their medicines, and where necessary checking patient files if they had permission. Medicines were recorded by brand or generic name, including prescription and non-prescription and over the counter, and all data were anonymised. Medications were coded using the World Health Organization (WHO) ATC classification system. 27 Two pharmacists (M.O.'D., J.P.) independently reviewed and confirmed medication entries.
Measuring exposure to anticholinergic medications
The dependent variable was participants' score for anticholinergic burden calculated using the updated 2012 ACB scale. 28 In addition, the ACB list was assessed and modified to include drugs with anticholinergic properties taken by participants, available in Ireland, but not included in the ACB scale. Reference Fox, Richardson, Maidment, Savva, Matthews and Smithard20 Two pharmacists (M.O.'D., I.M. – who developed the original scale) independently consulted standard reference sources, the product characteristics (SmPC) information and the other validated anticholinergic rating scales, to assign a score to other drugs with anticholinergic properties available in Ireland but not included in the ACB list; this was based on the approach used to develop the original scale. The 22 medicines not included in the original ACB list with respective scoring are listed in online Table DS1. Medications with anticholinergic properties that were not available in Ireland and/or not present in the data-set were excluded (42 medications).
We categorised exposure to anticholinergics in three ways: (a) the total ACB score of each individual, created by summing the score of each possible (ACB 1) or definite (ACB 2 or 3) anticholinergic; (b) a binary variable – those exposed to any anticholinergic medicine (ACB score ⩾1) and no anticholinergic exposure (ACB 0); and (c) a categorical variable – no exposure to anticholinergic medications (ACB 0), ACB score of 1–4, and ACB score of ⩾5, as in previous studies. Reference Fox, Richardson, Maidment, Savva, Matthews and Smithard20
Covariates
Covariates included gender, age (a categorical variable: 40–49 years, 50–64 years, 65+ years), level of intellectual disability, place of residence (independent, community group home or residential setting). Residential settings were defined as living arrangements where ten or more people share a single living unit or where the living arrangements are campus based. Community group homes are in a community setting with staff support for small groups (<10) of people with intellectual disabilities. Other covariates included any dementia (doctor's diagnosis of dementia, organic brain dysfunction, senility or serious memory impairment), polypharmacy (no polypharmacy 0–4 medicines; polypharmacy ⩾5 medicines). Participants/proxies reported if the participant had ever received a doctor's diagnosis of 12 chronic health conditions. Reference McCarron, Swinburne, Burke, McGlinchey, Carroll and McCallion23 Dementia, lung disease, stroke, cancer and liver disease had insufficient numbers (<5% prevalence) and were excluded from further multivariate analysis.
The relationship between anticholinergic exposure and indicators of anticholinergic adverse effects was examined; if the participant had reported fall(s) in the previous year, daytime dozing, constipation or physician-diagnosed chronic constipation and laxative use. Participants were also divided into those who were dentate or edentulous. Reference Mac Giolla Phadraig, McCallion, Cleary, McGlinchey, Burke and McCarron29
Statistical analyses
Descriptive statistics (percentages, medians (as the data were not normally distributed) and 95% confidence intervals) described the characteristics of the eligible study population.
We used univariate analysis to examine the associations between the dependant (anticholinergic exposure (ACB ⩾1) v. no exposure) and clinical and demographic variables. Here, for categorical variables chi-squared (χ2) tests for independence were used to test for a significant association between the three ACB groupings. For continuous variables, a one-way analysis of variance (ANOVA) was used to test for a significant difference. Multinomial logistic regression identified factors associated with an ACB of 1–4 and an ACB of 5+, with those with no anticholinergic exposure (ACB 0) as the reference category. All demographic variables were included in the model (age, gender and level of intellectual disabilities). Those with an unverified level of intellectual disabilities (n = 54) were excluded from regression analyses. Those who lived independently or in community group homes were combined as a single group, as the numbers in the independent setting with anticholinergic exposure were small (n = 11). Variables with a P<0.10 in univariate analysis were included in our multivariable model (this P-value was selected to ensure that important or influential factors were not omitted). Reference Tabachnick and Fidell30 All variables were entered into the model simultaneously. The model is adjusted for polypharmacy status (polypharmacy v. no polypharmacy), with results presented as adjusted odds ratios (ORs) with corresponding 95% confidence intervals.
Sample size calculation for the logistic regression was based on the guideline of Peduzzi et al (for a minimum number of cases (N) needed for the study; N = 10 k/p, where p is the smallest of the proportions of negative or positive cases in the population, k the number of covariates (independent variables)). Reference Peduzzi, Concato, Kemper, Holford and Feinstein31 For the regression model there were ten covariates and the proportion of negative cases (ACB 0) was 0.284, therefore a minimum sample size (N) of 352 was needed. There were data from 658 individuals available for regression analyses, so sample size was sufficient. The ACB scale score and anticholinergic adverse effects were explored at univariate level. To control for problems of Type I error associated with multiple comparisons a Bonferroni correction was applied, Reference Benjamini and Hochberg32 testing six associations, with a desired α of 0.05, resulting in α = 0.05/6 = 0.008. Statistical analyses were carried out using the Statistical Package for Social Sciences, Version 20.
Results
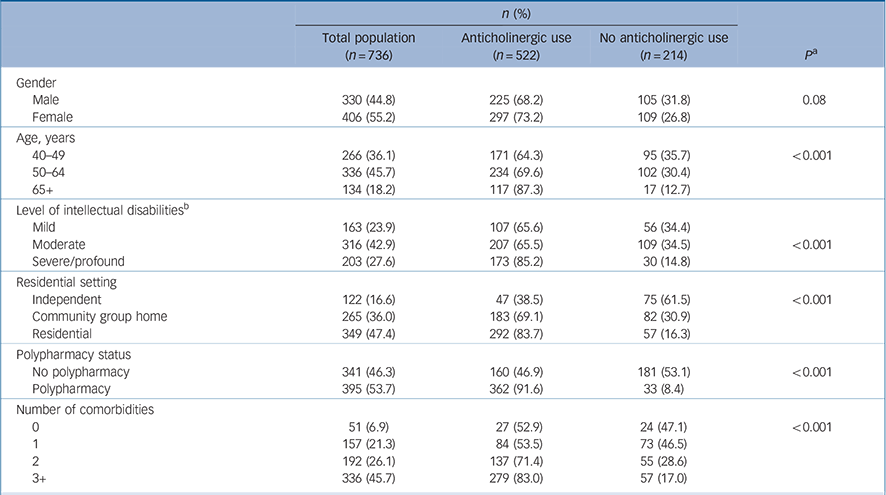
Of 753 participants, 736 (98%) provided medication use data. Baseline characteristics of our sample are presented in Table 1. The mean age of participants was 54.1 years (s.d. = 8.8, range 41–90 years), with almost half (45.7%) aged between 50 and 64 years. Almost half (46%) of the sample with recorded level of intellectual disabilities (n = 682) reported moderate intellectual disabilities. Overall, participants reported a mean of 5.7 (s.d. = 4.4) medicines, with 53.7% exposed to polypharmacy (5+ medicines). In the total sample of 736, no exposure was reported by 214 (29.1%, ACB 0) whereas 308 (41.8%) had a score of ACB 1–4 and 214 (29.1%) ACB score 5+ (online Table DS2). Of those reporting medications with ACB =1 score (522) 71% (370) received medicines with an ACB score of 2 or 3, and of those (n = 370), 43% (159) reported concurrent use of two or more ACB 2 or 3 drugs. The median total ACB score was 4.0 (s.d. = 3.0) (range 1–16; n = 522). There was a significant association between ACB score and reporting mental health conditions (n = 706) (P<0.001); 46.6% had ACB 5+, and a further 47.2% had a score of 1–4 (P<0.001).
Table 1 Anticholinergic exposure binary table
| n (%) | ||||
|---|---|---|---|---|
| Total
population (n = 736) |
Anticholinergic
use (n = 522) |
No anticholinergic
use (n = 214) |
P a | |
| Gender | ||||
| Male | 330 (44.8) | 225 (68.2) | 105 (31.8) | 0.08 |
| Female | 406 (55.2) | 297 (73.2) | 109 (26.8) | |
| Age, years | ||||
| 40–49 | 266 (36.1) | 171 (64.3) | 95 (35.7) | <0.001 |
| 50–64 | 336 (45.7) | 234 (69.6) | 102 (30.4) | |
| 65+ | 134 (18.2) | 117 (87.3) | 17 (12.7) | |
| Level of intellectual disabilities b | ||||
| Mild | 163 (23.9) | 107 (65.6) | 56 (34.4) | |
| Moderate | 316 (42.9) | 207 (65.5) | 109 (34.5) | <0.001 |
| Severe/profound | 203 (27.6) | 173 (85.2) | 30 (14.8) | |
| Residential setting | ||||
| Independent | 122 (16.6) | 47 (38.5) | 75 (61.5) | <0.001 |
| Community group home | 265 (36.0) | 183 (69.1) | 82 (30.9) | |
| Residential | 349 (47.4) | 292 (83.7) | 57 (16.3) | |
| Polypharmacy status | ||||
| No polypharmacy | 341 (46.3) | 160 (46.9) | 181 (53.1) | <0.001 |
| Polypharmacy | 395 (53.7) | 362 (91.6) | 33 (8.4) | |
| Number of comorbidities | ||||
| 0 | 51 (6.9) | 27 (52.9) | 24 (47.1) | <0.001 |
| 1 | 157 (21.3) | 84 (53.5) | 73 (46.5) | |
| 2 | 192 (26.1) | 137 (71.4) | 55 (28.6) | |
| 3+ | 336 (45.7) | 279 (83.0) | 57 (17.0) | |
a. P<0.05 is significant.
b. For level of intellectual disabilities n = 682, as the level was not verified in 54 individuals.
Similarly, level of intellectual disabilities was associated with anticholinergic exposure; 36.5% of those with severe/profound intellectual disabilities had an ACB score of 5+, compared with just 19.9% of those with mild intellectual disabilities (n = 682, P<0.001, online Table DS2). In total, 72 different anticholinergic medicines were reported in 1266 instances (online Table DS3); most were ACB 1 medications (52.1%) with 36.3% ACB 3 drugs.
Antipsychotics comprised 35.4% of the total cumulative ACB score, followed by anticholinergics (16%) (ATC N04A, for example biperiden) (Fig. DS2). Of those taking antipsychotics (319), 26% (n = 82) received two or more concurrently. Medications with ACB score 2 were reported by 26.6% of those with exposure, with carbamazepine being the most frequent (n = 127). ACB score 3 medicines were reported by 59.1% (n = 309), with olanzapine the most frequent (n = 101). Antipsychotics accounted for 46% of ACB 3 medicines, N04A anticholinergics (27.6%) and antidepressants (9.4%). Of those who reported N04A anticholinergics (n = 122), 91.7% reported concurrent use of antipsychotics with anticholinergic properties and of those receiving antipsychotics (319), 35.2% also received N04A anticholinergic agents, and of those with antipsychotic polytherapy (n = 82), over half (58.5%, n = 48) received a N04A anticholinergic.
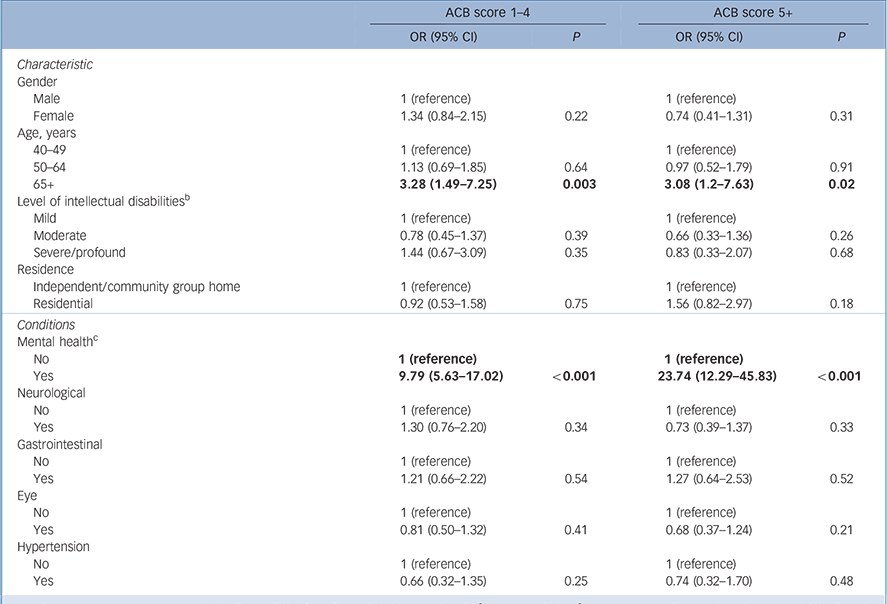
Those aged over 65 years were more likely to report an ACB score of 1–4 (OR = 3.28, 95% CI 1.49–7.28) and ACB of 5+ (OR = 3.08, 95% CI 1.20–7.63), after controlling for other factors (Table 2). Having a mental health condition was associated with having a score of ACB 1–4 (OR = 9.79, 95% CI 5.63–17.02) and ACB 5+ (OR = 23.74, 95% CI 12.29–45.83). Levels of intellectual disabilities, gender or place of residence were not significant with either level of anticholinergic exposure, nor were the other clinical conditions.
Table 2 Multivariate analysis of factors associated with Anticholinergic Cognitive Burden (ACB) scale scores of 1–4 and 5+ (n = 658) a
| ACB score 1–4 | ACB score 5+ | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Characteristic | ||||
| Gender | ||||
| Male | 1 (reference) | 1 (reference) | ||
| Female | 1.34 (0.84–2.15) | 0.22 | 0.74 (0.41–1.31) | 0.31 |
| Age, years | ||||
| 40–49 | 1 (reference) | 1 (reference) | ||
| 50–64 | 1.13 (0.69–1.85) | 0.64 | 0.97 (0.52–1.79) | 0.91 |
| 65+ | 3.28 (1.49–7.25) | 0.003 | 3.08 (1.2–7.63) | 0.02 |
| Level of intellectual disabilities b | ||||
| Mild | 1 (reference) | 1 (reference) | ||
| Moderate | 0.78 (0.45–1.37) | 0.39 | 0.66 (0.33–1.36) | 0.26 |
| Severe/profound | 1.44 (0.67–3.09) | 0.35 | 0.83 (0.33–2.07) | 0.68 |
| Residence | ||||
| Independent/community group home | 1 (reference) | 1 (reference) | ||
| Residential | 0.92 (0.53–1.58) | 0.75 | 1.56 (0.82–2.97) | 0.18 |
| Conditions | ||||
| Mental health c | ||||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 9.79 (5.63–17.02) | <0.001 | 23.74 (12.29–45.83) | <0.001 |
| Neurological | ||||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 1.30 (0.76–2.20) | 0.34 | 0.73 (0.39–1.37) | 0.33 |
| Gastrointestinal | ||||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 1.21 (0.66–2.22) | 0.54 | 1.27 (0.64–2.53) | 0.52 |
| Eye | ||||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 0.81 (0.50–1.32) | 0.41 | 0.68 (0.37–1.24) | 0.21 |
| Hypertension | ||||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 0.66 (0.32–1.35) | 0.25 | 0.74 (0.32–1.70) | 0.48 |
a. Reference category: ACB 0. P<0.05 is significant, all significant factors in bold Cox and Snell R 2 = 0.46, Nagelkirke R 2=0.52. Data are adjusted odds ratio (OR). Model is adjusted for polypharmacy status.
b. 54 no verified level of intellectual disabilities.
c. 30 missing data/do not know.
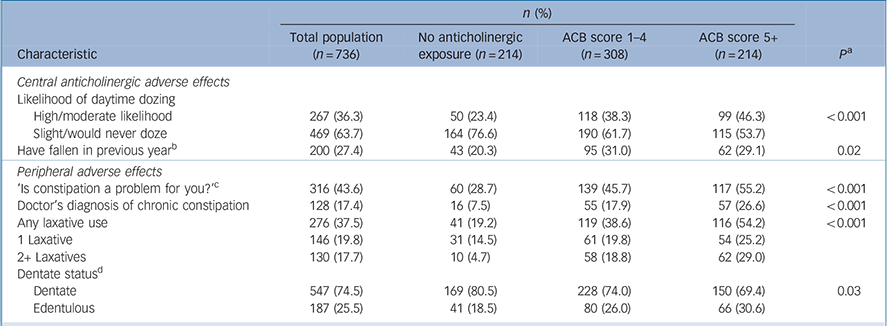
Daytime drowsiness was significantly associated with a higher ACB score at univariate level (P<0.001), with 46.3% of those with an ACB score of 5+ reporting a moderate/high likelihood of daytime drowsiness, compared with 23.4% of those with no anticholinergic exposure (Table 3). A greater proportion of those with higher anticholinergic burden reported a doctor's diagnosis of chronic constipation; 26.6% of those with an ACB score of 5+ compared with 7.5% of those with no anticholinergic exposure (P<0.001). Furthermore, 29.0% of those with an ACB 5+ used two or more concurrent laxatives, compared with 4.7% of those with no exposure (P<0.001).
Table 3 Anticholinergic Cognitive Burden (ACB) scale scores and adverse effects
| n (%) | |||||
|---|---|---|---|---|---|
| Characteristic | Total
population (n = 736) |
No
anticholinergic exposure (n = 214) |
ACB
score 1–4 (n = 308) |
ACB
score 5+ (n = 214) |
P a |
| Central anticholinergic adverse effects | |||||
| Likelihood of daytime dozing | |||||
| High/moderate likelihood | 267 (36.3) | 50 (23.4) | 118 (38.3) | 99 (46.3) | <0.001 |
| Slight/would never doze | 469 (63.7) | 164 (76.6) | 190 (61.7) | 115 (53.7) | |
| Have fallen in previous year b | 200 (27.4) | 43 (20.3) | 95 (31.0) | 62 (29.1) | 0.02 |
| Peripheral adverse effects | |||||
| ‘Is constipation a problem for you?’ c | 316 (43.6) | 60 (28.7) | 139 (45.7) | 117 (55.2) | <0.001 |
| Doctor's diagnosis of chronic constipation | 128 (17.4) | 16 (7.5) | 55 (17.9) | 57 (26.6) | <0.001 |
| Any laxative use | 276 (37.5) | 41 (19.2) | 119 (38.6) | 116 (54.2) | <0.001 |
| 1 Laxative | 146 (19.8) | 31 (14.5) | 61 (19.8) | 54 (25.2) | |
| 2+ Laxatives | 130 (17.7) | 10 (4.7) | 58 (18.8) | 62 (29.0) | |
| Dentate status d | |||||
| Dentate | 547 (74.5) | 169 (80.5) | 228 (74.0) | 150 (69.4) | 0.03 |
| Edentulous | 187 (25.5) | 41 (18.5) | 80 (26.0) | 66 (30.6) | |
a. From χ2-test (and applying Bonferroni correction), P<0.008 for significance.
b. Five missing data.
c. Eleven missing data.
d. Two missing data.
Discussion
Principal findings
As the first study in a representative population of older adults with intellectual disabilities, our findings reveal high levels of cumulative anticholinergic exposure, with 30% exposed to an ACB score of 5+. Multivariable regression analysis showed that those over 65 years and those with mental health conditions were much more likely to have high anticholinergic exposure. Antipsychotics, N04A anticholinergics, anti-epileptics and antidepressants were the most frequent classes contributing to the ACB. Antipsychotics accounted for over one-third of the cumulative burden, with a notably high prevalence of typical antipsychotics and with one in four of these individuals taking two or more antipsychotics. Our findings revealed that higher anticholinergic burden was associated with greater likelihood of reporting daytime dozing, constipation and use of multiple laxatives.
Comparison with other studies
There are no equivalent studies with other cohorts with participants with intellectual disabilities. Compared with studies that used the ACB scale with cohorts of older adults without intellectual disabilities, the degree of the anticholinergic burden found in our study was much greater and the types of anticholinergic drugs were different (online Table DS4). Reference Fox, Richardson, Maidment, Savva, Matthews and Smithard20,Reference Myint, Fox, Kwok, Luben, Wareham and Khaw33,Reference Richardson, Bennett, Maidment, Fox, Smithard and Kenny34
Factors associated with anticholinergic burden and adverse effects
Our analysis showed no association between higher scores and gender, but age over 65 years was a significant factor both for exposure to a score of 1–4 and exposure to an ACB score of five or more (Table 2). After adjusting for relevant confounders, we did not find an association between the level of intellectual disabilities and anticholinergic burden; however, it was notable that 85% of those with severe or profound intellectual disabilities were exposed to anticholinergic medications, and over one-third to an ACB score of 5+. The confidence intervals across all the categories were quite wide indicating the scale of variation remaining after adjusting for confounding factors, including polypharmacy. Other studies have only examined psychotropic polypharmacy Reference Tsiouris, Kim, Brown, Pettinger and Cohen35,Reference Stolker, Heerdink, Leufkens, Clerkx and Nolen36 or polypharmacy Reference Haider, Ansari, Vaughan, Matters and Emerson37 and have reported varying associations with age, gender and level of intellectual disabilities, but in contrast to the general elderly population, where women are identified as being more likely to be exposed to psychotropic polypharmacy. Reference Qato, Alexander, Conti, Johnson, Schumm and Lindau38 It may be that many of the conditions that are treated with anticholinergic medicines occur earlier in the lives of people with intellectual disabilities than in those without intellectual disabilities so that the use of these medicines has become similar in men and women and is increasing to a lesser extent in these older age groups.
Almost half of those with a mental health condition and four in ten of those over 65 were exposed to an ACB of 5+. The wide confidence intervals of the association of mental health conditions may reflect variability in reporting of mental health conditions; however, 12 of the 16 highest contributors to the ACB score were drugs for mental health. We found that 35% of those with antipsychotics had concurrent exposure to N04A anticholinergics, which was higher than previously reported in a UK study (14%), Reference Paton, Flynn, Shingleton-Smith, McIntyre, Bhaumik and Rasmussen39 yet Parkinson's disease was reported by only 1% in this cohort. The risks associated with using these medicines in combination in patients who are vulnerable and cognitively impaired are substantial. Reference Banerjee40 Our findings revealed that over one-fifth of those reporting antipsychotic use reported chlorpromazine and 14% reported haloperidol, both agents that carry significant anticholinergic, noradrenergic and antihistamine adverse effects. Reference Taylor, Paton and Kapur41 These older agents are associated with more extrapyramidal side-effects Reference Taylor, Paton and Kapur41 and people with intellectual disabilities may be more susceptible to these side-effects compared with the general population. Reference Bhaumik and Branford17,Reference Taylor, Paton and Kapur41 Risperidone was also the second most commonly used antipsychotic, an agent also associated with extrapyramidal side-effects. Reference Taylor, Paton and Kapur41 Although our findings are limited by the fact that we did not have information in relation to side-effects of medications, it is probable that these anticholinergic agents are being to some extent used to treat, or in prophylaxis of, extrapyramidal symptoms associated with antipsychotic agents. There is recent evidence of increased incident dementia associated with higher anticholinergic burden and length of exposure in those over 65 years. Reference Gray, Anderson, Dublin, Hanlon, Hubbard and Walker7
Our univariate findings must be interpreted conservatively; while a higher anticholinergic burden was associated with a risk of daytime dozing, falls in the previous year were not significantly associated, in contrast to studies in the general older population. Reference Ruxton, Woodman and Mangoni6,Reference Lu, Wen, Chen and Hsiao42 Constipation is common in older people, and increases with age. Reference Gallagher and O'Mahony43 People with intellectual disabilities are at risk of constipation from several factors, Reference Böhmer, Taminiau, Klinkenberg-Knol and Meuwissen44 and we found an association between increasing anticholinergic burden and constipation and laxative use, and furthermore, almost 30% of those with an ACB score of 5+ had laxative polytherapy, compared with <5% of those with no anticholinergic exposure. Multiple laxative use poses risks of electrolyte disturbance and dehydration that may exacerbate constipation. Reference Gallagher and O'Mahony43 The relationship between anticholinergic medications and xerostomia and tooth loss has been previously established Reference Gil-Montoya, de Mello, Barrios, Gonzalez-Moles and Bravo45 but although a higher proportion of the participants with an ACB score of 5+ were edentulous (30.6%), this was not significant.
Impact of findings on practice
Since anticholinergic activity may affect both central and peripheral systems, several factors make managing the anticholinergic burden complex in people with intellectual disabilities. Multimorbidity combined with complex mental health conditions and epilepsy increases the number and different classes of drugs with anticholinergic activity prescribed for people with intellectual disabilities and the cumulative burden. The sensitivity of people with intellectual disabilities to the effects of these drugs may be greater, and may increase with age, but is unquantifiable because of lack of evidence. Consequently, the prevalence of anticholinergic side-effects may be greater in this population, especially as the oldest age group were exposed to the greatest burden. Patient assessment is challenging, which may lead to diagnostic over-shadowing Reference Tyrer, Cooper and Hassiotis14 and initiation of inappropriate drugs. Physical problems, such as constipation, may present as challenging behaviours, Reference Taylor, Paton and Kapur41 which could trigger a prescribing cascade with a significant anticholinergic burden, as the association of antipsychotic, anticholinergic and laxative use in this study suggests.
A high proportion of people with intellectual disabilities are prescribed drugs with anticholinergic effects from an early age, and are likely to be exposed for many years. Reference Bhaumik and Branford17 Length of exposure may increase as life expectancy of people with intellectual disabilities grows, potentially increasing the risks of chronic use of psychotropics. Reference Gøtzsche, Young and Crace46 These factors imply that the extent and burden of anticholinergic side-effects in people with intellectual disabilities are greater than in the general older population, and could have an impact on their quality of life. Therefore, assessment of this burden, particularly among the oldest and those with mental health conditions and multiple morbidities, and who receive psychotropic polypharmacy is essential.
The risks of cumulative anticholinergic burden could be reduced through regular, multidisciplinary medication review. Scales such as the ACB scale, allied to review of patient symptoms, currently remain useful aids to guide clinical decision-making. Reference Gerretsen and Pollock47 Little is known about the influence of ageing on people with intellectual disabilities and their response to medicines, which reinforces the need for review and education of healthcare professionals. Integrated and coordinated care is receiving increased attention in the older population, Reference Oliver48 and needs to be further developed when providing care to people with intellectual disabilities. In older people and those with cognitive impairment, anticholinergic-induced cognitive impairment is more likely to occur at therapeutic doses potentially increasing risks of medication errors Reference Maidment, Haw, Stubbs, Fox, Katona and Franklin49 for people with intellectual disabilities managing their own medicines. Continuing deinstitutionalisation creates challenges for primary care professionals who may not have the necessary expertise or experience to provide care for people with intellectual disabilities, nor may they be able to meet the needs of their carers. Reference McCartney50 Guidelines are needed to support professionals, people with intellectual disabilities and carers to optimise anticholinergic medicines use. However, since people with intellectual disabilities are often excluded from randomised controlled trials, Reference Freedman51 additional data may also need to be generated by national audits and longitudinal studies. Reference Tyrer, Cooper and Hassiotis14
Strengths and limitations
Our study has four important strengths. First, use of a large, randomly selected population-representative sample offered sufficient power for multivariate analysis, with findings generalisable to the population with intellectual disabilities in Ireland. Second, the great majority of respondents recorded detailed medication data, including over-the-counter medicines (98%). Third, participants and/or proxy respondents underwent a detailed assessment of health characteristics, providing data on potential confounders for the regression model. The use of the Bonferroni correction addressed the problem of multiplicity. Fourth, we used the ACB scale, which has been widely used, making the assessment of anticholinergic burden robust and relevant to clinical practice. Reference Campbell, Boustani, Limbil, Ott, Fox and Maidment3,Reference Fox, Richardson, Maidment, Savva, Matthews and Smithard20 We added to its content validity by reviewing other anticholinergic medicines available in Ireland with an independent expert.
There are also limitations. There was no independent confirmation of medicines or conditions, but cross-checking of medicines in the pre-interview questionnaire at the time of interview improved accuracy. Information was also not recorded about disease severity. Data on dose and frequency of medicines were not always available and adverse effects may be dose dependent, Reference Gerretsen and Pollock47 however, the ACB scale does not take dose into account. Although a higher dose of an anticholinergic agent would be expected to cause more central effects, the relationship may not be linear. Reference Chew, Mulsant, Pollock, Lehman, Greenspan and Mahmoud52 The ACB scale has not been validated against measures of in vitro anticholinergic activity. However, assays are difficult to interpret, not readily available in practice and due to variations in blood–brain barrier permeability may not reflect levels in the central nervous system. It is currently accepted, that allied to a careful review of the patient's symptoms and medicines, scales and lists such as the ACB scale remain the best aid to guide clinical decision-making. Reference Gerretsen and Pollock47 The ACB scale does not take into account influences of patient variability in drug response associated with older age, frailty, multimorbidity, cognitive reserve and individual pharmacokinetic factors.
As an observational study, we could only describe associations between anticholinergic burden and clinical and demographic factors. In our multivariate analysis, potential bias was reduced by adjusting for known confounders; however, residual confounding may remain. Although potential adverse effects associated with anticholinergic exposure were examined at univariate level, other factors such as functional status, or baseline cognitive status that could influence the prescription of anticholinergics were not measured in this study, and this analysis was descriptive and not adjusted for confounders.
In conclusion, the use of medications with anticholinergic activity is commonplace among older adults with intellectual disabilities, with psychotropic agents accounting for much of the burden. For the first time in people with intellectual disabilities a high anticholinergic burden has been shown to be associated with daytime dozing, constipation and multiple laxative use. The possible impact that anticholinergics may have on cognitive and executive function should be evaluated and more attention should be paid to the assessment of peripheral anticholinergic effects, such as constipation. People with intellectual disabilities are among the most vulnerable members of society and regular, multidisciplinary review of medications to decrease the use of anticholinergic medicines is likely to reduce morbidity and improve quality of life in this population.
Funding
This research was funded by the Department of Health in Ireland and managed by the Health Research Board. The lead author (M.O.'D.) received funding for a PhD from the Trinity College Dublin Studentship. The funding body did not play a role in the study design or writing of the manuscript. The views expressed are those of the authors and are not necessarily those of the Department of Health, the Health Research Board or Trinity College Dublin.
Acknowledgements
We would like to thank the people with intellectual disabilities who participated in this study, their families, the services involved, the IDS-TILDA Scientific Advisory Committee and the Intellectual Disability Consultative Groups for their support. We would like to acknowledge the contributions of Dr Caoimhin MacGiolla Phadraig, Anne Belton, Professor John Haslett and Dr Rachael Carroll.








eLetters
No eLetters have been published for this article.