Unprecedented attention is being directed towards the treatment of children with serotonin reuptake inhibitors (SRIs) and other antidepressants, owing to concerns about potential increases in the risk of suicidal thoughts and perhaps suicide attempts, arising from randomised controlled trials of these drugs v. placebo. 1–Reference Friedman and Leon13 These concerns may be having an effect of reducing usage of SRIs as well as limiting new diagnoses of adult as well as juvenile depression. Reference Libby, Brent, Morrato, Orton, Allen and Valuck14–Reference Valuck, Libby, Orton, Morrato, Allen and Baldessarini16 Given the importance of rationally balancing the risks and benefits of antidepressant treatment of children and adolescents, we carried out a systematic meta-analysis aimed at critically evaluating the magnitude of efficacy of all types of antidepressants. We also compared treatment responses in children v. adolescents and with SRIs v. other types of antidepressants, and identified factors associated with measures of treatment efficacy.
Method
Search strategy and criteria
We searched computerised databases for published or unpublished trials in which any antidepressant was compared with placebo among participants aged 20 years or less and diagnosed with major depressive disorder. Sources included MEDLINE, PsycInfo, the Cochrane Central Register of Controlled Trials, PsiTri, EMBASE and ClinicalTrials.gov, each through May 2006, without language restrictions. In a further effort to locate studies, we examined reference lists of identified publications and reviews; consulted pharmaceutical company websites for unpublished trial results and contacted authors of identified reports to clarify methodological questions and to seek unpublished data that could be included; consulted experts in paediatric psychiatry and psychopharmacology in both the EU and USA; and searched the UK Committee on Safety of Medicines and the US Food and Drug Administration websites for relevant information. 1
The first author (E.M.T.) initially screened reports for potential usefulness, identifying placebo-controlled trials involving juvenile patients diagnosed with a depressive illness and treated with antidepressants. The first and second authors (E.M.T., F.S.) then applied the following inclusion criteria independently, and reached consensus with the other authors. The criteria were:
-
(a) prospective, parallel groups, double-blind trial design with random assignment to an antidepressant or placebo;
-
(b) meeting standard diagnostic criteria of DSM–III (or later) 17 for a depressive disorder (major depression, dysthymia or depression not otherwise specified) or of ICD–9/10 18 for depressive disorder, or diagnosis by clinical or structured diagnostic interview;
-
(c) participants aged 20 years or less;
-
(d) ‘responder’ rates reported as participant counts in each treatment arm (responders/participants exposed to drug or placebo, n/N) and not only as average percentage change in symptom rating scale scores.
Ultimately 29 of 304 initially screened studies met these criteria; Reference March, Silva, Petrycki, Curry, Wells, Fairbanks, Burns, Domino and McNulty5,Reference Kramer and Feiguine19–Reference Beecham44 Fig. 1 shows a summary of the study selection process as recommended by the Quality of Reporting of Meta-analyses (QUOROM) statement. Reference Moher, Cook, Eastwood, Olkin, Rennie and Stroup45 All 29 studies had antidepressant and placebo arms, and one included a third arm (placebo v. SRI v. tricyclic antidepressant), Reference Deas, Randall, Roberts and Anton38 to yield 30 drug–placebo contrasts for meta-analysis.

Fig. 1 Selection process for studies to be included in the meta-analyses reported.
Data extraction
First author E.M.T. extracted data from reports, which were verified independently by F.S.; discrepancies were resolved by consensus of all authors. For studies reported more than once, we used the most complete samples available. Information extracted included items required for meta-analysis of efficacy rate ratios and for meta-regressions outlined below. For continuous measures of clinical symptom rating scales we compiled a uniform percentage scale of clinical severity (maximum possible scale score set at 100%): for example, for the 21-item Hamilton Rating Scale for Depression the maximum score of 68 points was taken as 100%, so that entry scores of 20 or more were considered equivalent to percentage scores of 29.4% or over; other scale conversions are defined below. Definitions of ‘treatment responder’ varied between studies. Adherence to randomly assigned study medication and precise days of treatment exposures were reported inconsistently, but the percentage of patients retained was available for all studies.
We compared doses across agents and studies by use of standardised imipramine equivalent (IMIeq) daily doses, despite the lack of firm definitions of clinical dose–effect relationships with tricyclic antidepressants or SRIs at any age. The potency ratios selected were based on the empirical option of estimating median doses from manufacturers' recommendations, textbook summaries, and clinical practice with actually employed doses for each agent, derived largely from studies and clinical experience with adults. Reference Baldessarini, Brunton, Lazo and Parker46
Meta-analysis
‘Responder’ rate (proportion of sample attaining a study-specified level of improvement in clinical ratings, typically at least 50% of initial ratings of depressive symptom severity) was determined for the primary categorical outcome measure defined in each study (or that most consistent with other trials when a primary outcome was not specified) to distinguish responders (n) and non-responders (N–n). Statistical analyses employed Intercooled Stata software, version 9.2 for Windows (2006, Stata-Corp, College Station, Texas, USA). Trial results were combined through the Stata METAN command, using fixed-effects models to estimate pooled Cochran–Mantel–Haenszel drug:placebo rate ratios (RRs) and rate differences (RDs) and their 95% confidence intervals for all antidepressants combined, for each drug type considered separately (tricyclic antidepressants 14 trials; SRIs 12 trials; other drugs – mirtazapine, moclobemide, nefazodone – 4 trials), and for reported age groups (adolescents 16 trials; mixed ages 10 trials; children 4 trials). Random-effects models were also considered, based on tests for interstudy heterogeneity in selecting the reported pooled values, as recommended by DerSimonian & Laird. Reference DerSimonian and Laird47 A forest plot (rate ratio for each trial and a pooled value, with 95% confidence intervals, and symbol size proportional to study size and variance) summarised salient results graphically (Fig. 2).

Fig. 2 Forest plot of rate ratios (RR, with 95% CI) of responses to drug or placebo in 30 randomised double-blind placebo-controlled comparisons of rates of ‘response’ to antidepressants v. placebo, with overall pooled RR (1.22; 95% CI 1.15–1.31; blue diamond), based on meta-analysis.
Squares represent trials of serotonin reuptake inhibitors (SRIs; 12 trials); circles represent tricyclic antidepressants (TCAs; 4 trials) and other types of antidepressants (4 trials); the size of the data point is proportional to weight defined by study participant number and measurement variance.
The reported models underwent influence analyses, based on recalculating estimates of pooled rate ratios or rate differences after excluding individual trials serially, to identify those with unusually high influence on pooled estimates. Additional sensitivity analyses assessed effects of double inclusion of participants receiving placebo from a three-arm study. Reference Deas, Randall, Roberts and Anton38 We used Q and I Reference Jureidini, Doecke, Mansfield, Haby, Menkes and Tonkin2 statistics to test for heterogeneity, and computed pooled estimates by both Cochran–Mantel–Haenszel and inverse variance methods. We assessed potential publication bias with Begg's Reference Begg and Mazumdar48 and Egger's Reference Egger, Davey-Smith, Schneider and Minder49 tests, funnel plots (of the standard error of each trial rate ratio estimate v. each rate ratio, to test for symmetry of distribution of values above and below the computed pooled rate ratio at all observed levels of s.e.) and meta-regression analyses. We also reported the weighted number needed to treat (NNT) (or to harm, NNH) to obtain an antidepressant response from one additional patient, as estimated by the reciprocal of the fixed-effects rate differences and their 95% confidence intervals. Reference Altman50
Meta-regression
To evaluate study or participant characteristics associated with trial outcomes, we conducted meta-regression analyses of rate ratios v. the following study characteristics: drug type (tricyclic antidepressants v. SRIs v. other antidepressants); sample size; publication year; published v. unpublished; type of analysis – completer v. last observation carried forward (LOCF); funding source (industry-sponsored or not); exposure weeks; and estimated methodological quality of each study based on Jadad ratings (from 1 to a high score of 4). Reference Jadad, Moore, Carroll, Jenkinson, Reynolds, Gavaghan and McQuay51 Additional patient characteristics considered were age group (children, mixed ages, adolescents); average drug dose (approximate equivalent to imipramine in mg/day, based on a typical daily dose of 150 mg/day for imipramine); Reference Baldessarini, Brunton, Lazo and Parker46 in-patient/out-patient status; and baseline illness severity (percentage of maximum scale score required for trial entry). Variables were considered further based on their ability to alter the estimated pooled rate ratio by 0.10 or more. Based on preliminary univariate regressions to support ranking their effects on rate ratio, we then introduced variables into a meta-regression model in descending rank order.
Results
Search findings
The search strategy yielded 304 non-duplicative citations, from which 90 pertained to treating juvenile depression (Fig. 1). Of these, 37 were open trials, anecdotal case series or reviews, and 7 of the remaining 53 did not meet full inclusion criteria. In 17 of the 46 trials remaining, specific responder rates (n/N) were not provided or data were reported in preliminary versions of included studies, leaving 29 trials (with 33 treatment arms). We also excluded the alprazolam arm of one study, Reference Bernstein, Garfinkel and Borchardt22 and two arms involving unmasked psychotherapy with or without fluoxetine in another. Reference March, Silva, Petrycki, Curry, Wells, Fairbanks, Burns, Domino and McNulty5 Of the remaining 29 studies, 5 were reported as abstracts or summaries from meetings, the US Food and Drugs Administration website or corporate websites. Reference Klein, Mannuzza, Koplewicz, Tancer, Shah, Liang and Davies35,Reference Milin, Simeon and Spenst37,Reference Keller, Ryan, Strober, Klein, Kutcher, Birmaher, Hagino, Koplewicz, Carlson, Clarke, Emslie, Feinberg, Geller, Kusumakar, Papatheodorou, Sack, Sweeney, Wagner, Weller, Winters, Oakes and McCafferty39,Reference Rynn, Findling, Emslie, Marcus, Fernandes, D'Amico and Hardy41 One report included data from two trials of the atypical antidepressant mirtazapine, Reference Keller, Ryan, Strober, Klein, Kutcher, Birmaher, Hagino, Koplewicz, Carlson, Clarke, Emslie, Feinberg, Geller, Kusumakar, Papatheodorou, Sack, Sweeney, Wagner, Weller, Winters, Oakes and McCafferty39 and another contained results from two trials of the SRI-like agent venlafaxine. Reference Beecham44 In summary, there were 30 contrasts arising from 29 randomised controlled trials for meta-analysis.
Characteristics and quality assessment of included studies
A total of 3069 participants were represented, not counting 91 placebo-treated participants in the study by Keller et al Reference Deas, Randall, Roberts and Anton38 considered twice in placebo comparisons with an SRI or tricyclic antidepressant (online Table DS1). The weighted mean participant age was 13.5 years (s.d.=0.4, median=14.5, range 6–20). Most trials with both children and adolescents did not report response rates for each age group separately. Only 4 studies (all involving tricyclic antidepressants) separately considered 121 (3.9%) children aged 12 years or less, Reference Petti and Law20,Reference Puig-Antich, Perel, Lupatkin, Chambers, Tabrizi, King, Goetz, Davies and Stiller21,Reference Hughes, Preskorn, Wrona, Hassanein and Tucker24,Reference Geller, Cooper, Graham, Fetner, Marsteller and Wells27 so that randomised controlled trials of modern antidepressants involving only or separately reported children with depression were not available. Adolescent participants (n=1427) were considered separately in the majority of comparisons (16 of 30 trials, 53%), representing 46.5% of all participants. Ten other samples (33%) included both adolescents and children (n=1521), representing 49.6% of participants. Information about gender-specific treatment effects was not provided in most reports.
Of 30 drug–placebo comparisons considered (Table DS1), 14 involved tricyclic antidepressants, including amitriptyline, clomipramine, desipramine, imipramine and nortriptyline; 12 involved SRI-type agents, including R,S-citalopram, fluoxetine, paroxetine, sertraline and venlafaxine; 4 involved other antidepressants, including the reversible type A monoamine oxidase inhibitor (MAOI–A) moclobemide and atypical agents mirtazapine and nefazodone. One trial involved three arms, with paroxetine or imipramine v. placebo, Reference Deas, Randall, Roberts and Anton38 and another included adolescents with depression and comorbid alcohol misuse. Reference Avci, Diler, Kibar and Toros36
In addition to being randomised, double-blind and placebo-controlled, all trials included use of modern standardised diagnostic criteria (Research Diagnostic Criteria for major depressive disorder, Reference Spitzer, Endicott and Robins52 DSM–III or later, or ICD–9/10) to diagnose clinical depression – major depressive disorder in 89.7% of cases. All studies except the earliest SRI trial Reference Simeon, Dinicola, Ferguson and Copping25 were considered adequate with respect to randomisation and masking. Quality ratings averaged 2.97 (s.d.=0.76, median=3.0, range 1–4), Reference Jadad, Moore, Carroll, Jenkinson, Reynolds, Gavaghan and McQuay51 and proportions of trials with high-quality scores (3 or 4) ranked tricyclic antidepressants (12/14 trials, 86%) > other antidepressants (3/4 trials, 75%) > SRIs (8/12 trials, 67%; online Tables DS1 and DS2). Outcomes were given for participants who completed the trials in only 6 studies; Reference Simeon, Dinicola, Ferguson and Copping25,Reference Boulos, Kutcher, Marton, Simeon, Ferguson and Roberts26,Reference Emslie, Rush, Weinberg, Kowatch, Hughes, Carmody and Rintelmann30,Reference Klein, Mannuzza, Koplewicz, Tancer, Shah, Liang and Davies35,Reference Milin, Simeon and Spenst37,Reference Deas, Randall, Roberts and Anton38 the other 23 involved LOCF-based outcomes (Table DS1). Given the scarcity of ‘completer’ analyses and the possible superiority of LOCF analysis, in order to enhance consistency and maximise the sample available for analysis, the reported results are based on LOCF outcomes.
Drug doses and exposure times
All studies included provided drug doses consistent with contemporary paediatric practice, based on body-weight-adjusted daily dosing (mg/kg) considered standard for treating adult major depressive disorder. Reference Baldessarini, Brunton, Lazo and Parker46 The overall IMIeq drug dose averaged 165 mg/day (s.d.=48). Median doses (25th–75th percentile values) for the three major drug classes ranked tricyclic antidepressants 200 (140–220) mg/day > other antidepressants 178 (156–180) mg/day > SRIs 132 (106–161) mg/day (Table DS2). By age groups, median (25th–75th percentile) doses ranked adolescents 200 (139–212) mg/day > mixed ages 160 (102–168) mg/day > children 140 (100–144) mg/day, suggesting similar mg/kg daily dosing.
Reported nominal trial durations yielded a weighted (by N/study) average exposure time of 8.7 weeks (median=8, range 1–12), indicating an overall trial experience of approximately 512 person-years. Withdrawal rates and actual exposure times are not described for most trials, but drug treatment averaging approximately 8 weeks should be adequate to detect antidepressant effects, at least based on studies in adult depression. Reference Baldessarini, Brunton, Lazo and Parker46 The studies analysed included three relatively brief trials – a 5-week trial of a tricyclic antidepressant, Reference Puig-Antich, Perel, Lupatkin, Chambers, Tabrizi, King, Goetz, Davies and Stiller21 another of a short-acting MAOI, Reference Birmaher, Waterman, Ryan, Perel, McNabb, Balach, Beaudry, Nasr, Karambelkar, Elterich, Quintana, Williamson and Rao34 and a 1-week trial of a tricyclic antidepressant. Reference Emslie, Heiligenstein, Wagner, Hoog, Ernest, Brown, Nilsson and Jacobson31 Total exposures (median) differed substantially between drug types, ranking SRIs 1584 person-weeks > other antidepressants 1000 person-weeks > tricyclic antidepressants 248 person-weeks (Table DS2). This imbalance of both exposure times and participant counts indicates major mismatching, tending to favour SRIs over tricyclic antidepressants, whereas median estimated IMIeq daily doses of SRIs were 34% lower than those of tricyclic antidepressants (Table DS2). Among age groups, median exposures ranked: adolescents and children 1148 person-weeks > children 281 person-weeks > adolescents 262 person-weeks. When both dose (IMIeq mg/day) and average exposure time (weeks) are considered together, there is no overall difference between drug types: SRIs mean=1232 mg-weeks (s.d.=269); tricyclic antidepressants mean=1239 mg-weeks (s.d.=642); other antidepressants mean=1257 mg-weeks (s.d.=339); one-way analysis of variance (ANOVA), F=0.004, d.f.=2,27, P=0.996.
Initial depression severity
We used a uniform percentage scale to represent initial illness severity across rating methods. Maximum scores on the 21-item Hamilton Rating Scale for Depression (HRSD; maximum score 68), the 17-item HRSD (maximum score 55), the Montgomery–Åsberg Depression Rating Scale (maximum score 60), the Psychiatric Rating Scale (maximum score 16), the School-Aged Depression List Interview (maximum score 119), the Children's Depression Rating Scale (maximum score 63), the Children's Depression Rating Scale – Revised (maximum score 113) and the Children Depression Inventory (maximum score 100) were all rated as 100%. Based on such normalised scaling, initial illness severity ratings were similar across placebo (mean=46.4, s.d.=15.9, median=49.9) and antidepressant (mean=47.5, s.d.=16.7, median=49.6) arms, indicating moderate to severe intensity of baseline depressive symptoms in most trials, all of which involved young people receiving out-patient treatment for depression. These initial depression ratings for participants randomised to drugs or placebo did not differ appreciably across drug types (Table DS2).
Treatment efficacy
We defined primary outcomes for meta-analysis a priori as responder status, based on changes in clinical ratings from intake to last observation point (as defined in each trial) involving substantial improvement, typically a 50% or greater reduction in symptomatic ratings of depression on standardised scales (Table DS1). Data pertaining to other outcomes of potential interest, including changes in continuous measures of depression severity, rates of clinically significant improvements or syndromal remission, and withdrawal rates, were reported too inconsistently to support systematic analysis.
Preliminary consideration of median response rates indicated modest differences between antidepressants and placebo, averaging 10.9% overall (60.1% with antidepressants v. 49.2% with placebo), and ranging from a difference of 8.0% for SRIs to 12.3% for tricyclic antidepressants (Table DS2). These average contrasts also were similar between age groups, ranging from 8.0% in adolescents to 16.2% in children, with a plausible intermediate difference of 11.6% in mixed-age juveniles (one-way ANOVA: F =0.304, d.f.=2,27, P=0.740; not shown), suggesting greater drug response with brain maturation.
Comparative efficacy of treatment with an antidepressant v. placebo, based on responder rates (i.e. proportions), is shown for all 30 available contrasts, as response rate ratios and rate differences and their 95% confidence intervals, based on meta-analysis (Fig. 2, Table DS3). Most trials (22/30 trials, 73%) yielded rate ratios of 1.0 or above, but only 6 (20%) met the criterion that the lower limit of the confidence interval of the trial rate ratio should be >1.0, and 5 of these involved an SRI – that is, fully 80% of comparisons (24/30) failed to meet this criterion of superiority of antidepressant over placebo, reflecting small effect differences.
An estimate of pooled overall effect size, based on meta-analysis to determine a pooled rate ratio and its confidence interval for all 30 trials, yielded a value of 1.22 (95% CI 1.15–1.31), indicating moderate (22%) overall superiority of antidepressant over placebo response rate on the multiplicative scale (Fig. 2, Table 1). Preliminary testing for interstudy variance (Q) Reference Begg and Mazumdar48 in this (P=0.28) and other meta-analyses (Table 1) supported use of a fixed-effects meta-analysis model. However, random effects modelling yielded a similar overall pooled rate ratio of 1.20 (95% CI 1.11–1.29).
Table 1 Meta-analytic comparisons of pooleda drug–placebo differences in response rates in subgroups in randomised trials of antidepressants for juvenile depression
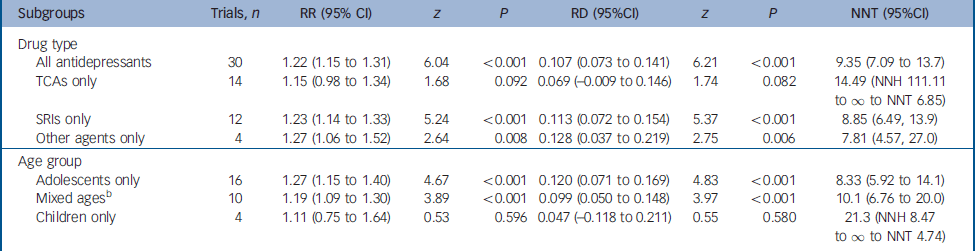
| Subgroups | Trials, n | RR (95% CI) | z | P | RD (95%CI) | z | P | NNT (95%CI) |
|---|---|---|---|---|---|---|---|---|
| Drug type | ||||||||
| All antidepressants | 30 | 1.22 (1.15 to 1.31) | 6.04 | <0.001 | 0.107 (0.073 to 0.141) | 6.21 | <0.001 | 9.35 (7.09 to 13.7) |
| TCAs only | 14 | 1.15 (0.98 to 1.34) | 1.68 | 0.092 | 0.069 (-0.009 to 0.146) | 1.74 | 0.082 | 14.49 (NNH 111.11 to ∞ to NNT 6.85) |
| SRIs only | 12 | 1.23 (1.14 to 1.33) | 5.24 | <0.001 | 0.113 (0.072 to 0.154) | 5.37 | <0.001 | 8.85 (6.49, 13.9) |
| Other agents only | 4 | 1.27 (1.06 to 1.52) | 2.64 | 0.008 | 0.128 (0.037 to 0.219) | 2.75 | 0.006 | 7.81 (4.57, 27.0) |
| Age group | ||||||||
| Adolescents only | 16 | 1.27 (1.15 to 1.40) | 4.67 | <0.001 | 0.120 (0.071 to 0.169) | 4.83 | <0.001 | 8.33 (5.92 to 14.1) |
| Mixed agesb | 10 | 1.19 (1.09 to 1.30) | 3.89 | <0.001 | 0.099 (0.050 to 0.148) | 3.97 | <0.001 | 10.1 (6.76 to 20.0) |
| Children only | 4 | 1.11 (0.75 to 1.64) | 0.53 | 0.596 | 0.047 (-0.118 to 0.211) | 0.55 | 0.580 | 21.3 (NNH 8.47 to ∞ to NNT 4.74) |
Serotonin reuptake inhibitors yielded a higher response rate ratio (RR=1.23, 95% CI 1.14–1.33) than tricyclic antidepressants (RR=1.15, 95% CI 0.98–1.34), but the overlapping confidence intervals indicate statistical non-separation by drug type. Of the SRIs, in its four trials (Table DS1) fluoxetine showed somewhat greater pooled efficacy than in eight trials of other SRIs: respectively, RR=1.45, 95% CI 1.24–1.70, range 1.00–1.74 v. RR=1.16, 95% CI 1.06–1.27, range 0.50–1.53; rate difference RD=0.196, 95% CI 0.117–0.276 v. RD=0.083, 95% CI 0.034–0.131; NNT=5.8, 95% CI 4.0–10.8 v. NNT=12.2, 95% CI 7.7–29.4. The largest effect size for fluoxetine was reported by March et al (RR=1.74, 95% CI 1.29–2.34), Reference March, Silva, Petrycki, Curry, Wells, Fairbanks, Burns, Domino and McNulty5 whereas its three remaining trials (Simeon et al, Reference Simeon, Dinicola, Ferguson and Copping25 RR=1.00; Emslie et al, Reference Emslie, Rush, Weinberg, Kowatch, Hughes, Carmody and Rintelmann30 RR=1.68; Emslie et al, 40 RR=1.27; Table DS3) yielded pooled effects (RR=1.32, 95% CI 1.10–1.59) similar to other antidepressants (overall RR=1.22, 95% CI 1.15–1.31). Other types of antidepressants tested in a small number of trials yielded a slightly higher pooled rate ratio (RR=1.27, 95% CI 1.06–1.52) than did the SRIs (Table 1), and again the 95% confidence intervals overlapped, and the weighted NNT estimates confirmed such overlap of efficacy. For all 30 trials, the NNT was 9.35 (95% CI 7.09–13.7); for tricyclic antidepressants the NNT was 14.49 (NNH 111.11 to ∞ to NNT 6.85, to manage the rate difference in the 95% CI range falling below zero); for SRIs the NNT was 8.85 (95% CI 6.49–13.9); and for other types of antidepressants it was 7.81 (95% CI 4.57–27.0).
Effects of age
The three age groups yielded an interesting progression of rising pooled rate ratio values with increasing age: children (RR=1.11, 95% CI 0.75–1.64) < juveniles of mixed ages (RR=1.19, 95% CI 1.09–1.30) < adolescents (RR=1.27, 95% CI 1.15–1.40; Table 1), with similar increases in rate differences and corresponding decreases of NNT by age (about 21, 10 and 8 respectively). For comparison with results from randomised placebo-controlled trials of antidepressants in adults, we applied meta-analysis to findings from over 250 trials involving 23 500 participants. Reference Janicak, Davis, Preskorn and Ayd54,Reference Khan, Detke, Khan and Mallinckrodt55 For adults, the overall pooled, random-effects rate ratio was 1.85 (95% CI 1.67–2.04; z=12.3, P<0.001) and the overall rate difference was 0.288 (95% CI 0.276–0.300; z=46.2, P<0.001), with an estimated NNT of 3.5 (95% CI 3.3–3.6), and results were similar for the different types of antidepressants (not shown). These findings add to the impression that antidepressant efficacy may increase with age.
Reporting bias and influence of individual trials
A funnel plot (s.e. of RR v. RR) encompassing published and unpublished trials indicated somewhat asymmetrical distribution of individual trial rate ratios leaning towards the positive about the pooled value of 1.22 as a function of the variance (s.e.) of individual trial RRs (RR values >1.22 averaged 0.42, s.e.=0.47 v. <1.22 averaged 0.27, s.e.= 0.23, a 1.54-fold difference) (not shown), and in meta-regression analyses the rate ratio was 22.0% (95% CI 1.5–41.8) higher in published v. unpublished trials, although unadjusted Begg's (P=0.34) and Egger's (P=0.30) tests did not indicate publication bias. We could not determine whether additional trials failing to find superior effects of antidepressants over placebo exist as unreported findings. Nevertheless, the preceding observations suggest that publication might have been biased towards studies favouring drug over placebo, perhaps tending to inflate available efficacy estimates.
Based on sensitivity or influence analysis, excluding one trial at a time and recalculating pooled rate ratio estimates, we found only trivial effects on the pooled rate ratio, including omission of the shortest trial, Reference Emslie, Heiligenstein, Wagner, Hoog, Ernest, Brown, Nilsson and Jacobson31 which accordingly was included in the overall analyses. The US federal grant-supported Treatment for Adolescents with Depression study by March et al Reference March, Silva, Petrycki, Curry, Wells, Fairbanks, Burns, Domino and McNulty5 was identified as the most influential study, based on its unusually large effect size (RR=1.74, 95% CI 1.29–2.34) and larger sample size (n=221) than in any other trial (Table DS3). Excluding this single trial reduced the overall pooled rate ratio to 1.20 (95% CI 1.12–1.28) from the overall rate ratio of 1.22.
Meta-regression analyses
Characteristics associated with a change in estimates of treatment effect (rate ratio) of at least 0.10 included outcome based on protocol completion v. LOCF methods; greater illness severity at entry; and published v. unpublished reports. Rate ratio estimates increased by approximately 10% (95% CI −13.4 to 31.8) for each 20% increase in depression severity ratings at intake. The less common method of ‘completer’ analysis (only 6 of 29 trials) was associated with a nearly 20% (95% CI −11.6 to 51.3) greater rate ratio than LOCF-based methods. Other factors considered (drug type, sample size, publication year, funding source, exposure weeks, estimated study methodological quality ratings, age group, average drug dose and in-patient or out-patient status) had only weak associations with drug/placebo response rate ratios. By adjusting the computed pooled response rate ratios for all three most influential covariates, the rate ratio difference between ‘older’ and ‘newer’ antidepressants persisted, but was even smaller (0.19) than without such adjustment (0.22). Specifically, these considerations fail to support the possibility that SRIs may be superior in efficacy to tricyclic antidepressants.
Discussion
The main findings in this meta-analytic review are that antidepressants have shown limited clinical efficacy in depression in adolescents, less among juvenile patients of mixed ages, and are poorly studied, but possibly even less effective, in children, and substantially less effective in juveniles than in adults. Reference Baldessarini, Brunton, Lazo and Parker46,Reference Fawcett and Barkin53–Reference Khan, Detke, Khan and Mallinckrodt55 On average, in juvenile participants with depression, drug and placebo response rates differed by only 10% and the pooled risk ratio was modest at 1.22 (Tables DS1, DS2). Drug and placebo response rates also differed little between drug types, and potential gender differences were indeterminate. The mg/kg body weight daily doses of antidepressants employed in the trials analysed were similar to those considered typical for adults with depression. Reference Baldessarini, Brunton, Lazo and Parker46
In line with recent British advisory recommendations, 56 this systematic review found limited efficacy for all types of antidepressants in short-term randomised controlled trials in juvenile depression. Earlier reviews of treatment of juvenile depression concluded that tricyclic antidepressants were ineffective, particularly in prepubertal children, with marginal efficacy in adolescents. Reference Hazell, O'Connell, Heathcote, Robertson and Henry57–Reference Jureidini, Doecke, Mansfield, Haby, Menkes and Tonkin59 Reviews of SRIs also have suggested limited antidepressant benefits in cases of juvenile depression. Reference Bridge, Iyengar, Salary, Barbe, Birmaher, Pincus, Ren and Brent12,Reference Jureidini, Doecke, Mansfield, Haby, Menkes and Tonkin59–Reference Raz65 Seemingly consistent with selective UK and US regulatory approval of fluoxetine for juvenile depression, there is some evidence of its apparent superiority to other SRIs, although this impression rests heavily on one large study with an unusually large drug–placebo separation. Reference March, Silva, Petrycki, Curry, Wells, Fairbanks, Burns, Domino and McNulty5 Conceivably the unusually long duration of action of fluoxetine Reference Baldessarini, Brunton, Lazo and Parker46 might be advantageous among treatment-reluctant and poorly treatment-adherent young patients. That antidepressants, and particularly SRIs, have mood-elevating effects in children and young adolescents is suggested by the substantial risk of these patients becoming excited or manic during treatment for apparent major depression. Reference Baldessarini, Faedda and Hennen66 Moreover, the modest evidence of efficacy in randomised controlled trials is at variance with the widespread empirical acceptance of modern antidepressants in paediatric clinical practice. Reference Zito, Safer, dosReis, Gardner, Boles and Lynch67–Reference Delate, Gelenberg, Simmons and Motheral69
A striking finding in our analysis is the limited difference found between outcomes with SRIs v. tricyclic antidepressants: with raw median response rates actually tended to favour the latter drugs, and meta-analytically pooled rate ratios differed little (7.7%), with highly overlapping confidence intervals, indicating statistical non-difference (Table DS2). Superiority of antidepressants over placebo in juvenile patients with major depressive disorder, based on statistical significance, was found in only 20% of 30 comparisons, including 0/14 comparisons involving tricyclic antidepressants, 0/4 involving other antidepressants, and only 5/12 comparisons involving SRIs (Table DS1). However, this way of comparing study results is confounded by the severe mismatching of participant numbers and treatment duration in trials of tricyclic antidepressants v. SRIs: notably, trials of SRIs had over six times greater treatment exposure in person-weeks (Table DS2). Greater statistical power gained with higher participant counts can yield ‘significant’ separation of drug from placebo, even though such separation may be small and of questionable clinical significance. Recent trials have been much larger (and longer) and so more likely to yield statistical separation of SRIs v. placebo, but without either a clinically or statistically meaningfully larger pooled effect size (rate ratio or rate difference) or smaller NNT in association with larger participant counts, as noted above. This mismatching of statistical power and exposure time (favouring SRIs) is to be contrasted with a 34% lower estimated equivalent daily dosing with SRIs than tricyclic antidepressants (Table DS2), which had a negligible effect on meta-regression outcomes. Nevertheless, in depressed patients of any age at high risk of suicidality, routine use of tricyclic antidepressants cannot be recommended in view of their known cardiotoxic effects and associated mortality in overdose.
Proposed explanations for such disappointing findings in the experimental therapeutics of juvenile depression include both methodological aspects of trial design and clinical aspects of juvenile mood disorders. Possibilities include relatively high rates of response to placebo or other non-specific interventions; age-inappropriate or insufficiently sensitive outcome measures; inadequately powered trials; adverse case selection (e.g. minimally ill, uncertain or heterogeneous diagnoses, previous treatment failures, poorly cooperative participants); incomplete control of substance misuse; inadequate dosing or duration of treatment; and simple lack of efficacy in juvenile v. adult mood disorders. Reference Courtney60,Reference Ryan63,Reference Birmaher, Ryan, Williamson, Brent, Kaufman, Dahl, Perel and Nelson70–Reference Timimi73 The average 60% drug response rate in juvenile depressed patients (Tables DS1, DS2) was similar to that reported in depressed adults, Reference Baldessarini, Brunton, Lazo and Parker46,Reference Fawcett and Barkin53,Reference Khan, Detke, Khan and Mallinckrodt55 suggesting that neither the major developmental changes in drug disposition Reference Biederman, Faraone, Baldessarini, Flood, Meyer, Wilens, Spencer, Chen and Weber74,Reference Wilens, Cohen, Biederman, Abrams, Neft, Faird and Sinha75 nor possible changes in pharmacodynamics are involved in the limited superiority of antidepressants to placebo in juvenile depression.
Poor separation in responses to antidepressants v. placebo may reflect recruitment of juveniles with relatively heterogeneous illnesses, who may meet nominal diagnostic criteria for major depressive disorder, but whose symptoms may not be representative of classic disorders, such as the clearly endogenous and melancholic features associated with adult major depression. Juvenile major depressive disorder was not accepted into the official American diagnostic nomenclature until the late 1990s. 17,Reference Wolraich, Felice and Drotar76,77 Differentiation of mood disorders from other prevalent behavioural and emotional conditions in young patients remains descriptive. Improved diagnostic and clinical rating methods may increase reliability of the diagnosis of major depressive disorder in children and adolescents, although not necessarily assure valid comparison with major depressive syndromes of adults. Reference Birmaher, Ryan, Williamson, Brent, Kaufman, Dahl, Perel and Nelson70,Reference Birmaher, Ryan, Williamson, Brent and Kaufman71,Reference Birmaher and Heydl78 Even though median baseline depression severity scores were at the mid-range of scale scores (averaging 50%; Table DS2), suggesting substantial illness severity, sampling of young patients with depression may include a high proportion of relatively mildly ill patients, who have most probably never received in-patient treatment, and who are more likely to improve spontaneously with or without additional effects of placebo treatment. Reference Hrobjartsson and Gotzsche79
Importantly, prepubertal children with depression may differ biologically from adolescents or adults, Reference Baldessarini, Faedda and Hennen66,Reference Biederman, Faraone, Baldessarini, Flood, Meyer, Wilens, Spencer, Chen and Weber74,Reference Kaufman, Martin, King and Charney80 and it remains unclear whether depression in prepubertal children is a substantially different disorder from that found in adolescents or adults, perhaps including developmental differences in either the pharmacodynamics of antidepressants or in their clinical effects. Reference Baldessarini, Faedda and Hennen66,Reference Martin, Kaufman and Charney81 The current data preclude adequate assessment of potential developmental effects owing to the paucity of studies of children considered separately. Only 4 of 30 treatment comparisons and 4% of participants involved children only; all were treated with tricyclic antidepressants, none with modern drugs. Nevertheless, within the limits imposed by the few studies of children, no major systematic difference was apparent between depressed children and adolescents in average rates of clinical improvement during treatment with either antidepressants or placebo, nor in pooled rate ratios (Table 1).
Implications
The limited benefits of antidepressant treatment for depression in children and adolescents found in reported results of randomised controlled trials have important risk–benefit implications, given current discussion of risks that may include more suicidal thinking and perhaps self-injurious behaviour in juvenile patients with depressive or anxiety disorders during treatment with an SRI. Clearly, additional research is required to clarify the basis of the limited juvenile responses to antidepressant treatments considered standard in adult major depression, to specify developmental changes in antidepressant potency and pharmacodynamics as well as potential age-related differences in treatment responses and risks involved. Moreover, questions of risks v. benefits of antidepressant treatment of juvenile depression require critical assessments of the relative value of modest effect sizes, typically based on 50% reductions in symptom ratings as reviewed here, v. actual clinical effectiveness (efficacy plus acceptability sufficient to yield clinical recovery), which remains to be studied in children and adolescents. Outcomes in future trials might be enhanced by including larger samples, more severely depressed participants, more children and narrower age groups. Trials should also include information on the proportion of individuals with mild or moderate depression at baseline. Moreover, results of all well-designed trials should be made publicly available regardless of outcome, since publication bias may affect available meta-analytical estimates of efficacy or adverse effects on which clinical therapeutic practices and public health policies are based. Urgent goals should include development of more effective, safe, cost-effective and accessible short-term and long-term treatments for juvenile depression, with specific consideration of differences between prepubertal children and adolescents.
Acknowledgements
The authors thank the Royal College of Psychiatrists, for awarding an Eli Lilly Travelling Fellowship to E.M.T.; the PhD programme in Psychiatry of the University of Pisa, the Harvard School of Public Health doctoral programme in Epidemiology and the IDEA Foundation, Milan for supporting F.S.; and the Bruce J. Anderson Foundation and the McLean Private Donors Psychopharmacology Research Fund for research grants to R.J.B.








eLetters
No eLetters have been published for this article.